Explore Jobs
- Jobs Near Me
- Remote Jobs
- Full Time Jobs
- Part Time Jobs
- Entry Level Jobs
- Work From Home Jobs
Find Specific Jobs
- $15 Per Hour Jobs
- $20 Per Hour Jobs
- Hiring Immediately Jobs
- High School Jobs
- H1b Visa Jobs
Explore Careers
- Business And Financial
- Architecture And Engineering
- Computer And Mathematical
Explore Professions
- What They Do
- Certifications
- Demographics
Best Companies
- Health Care
- Fortune 500
Explore Companies
- CEO And Executies
- Resume Builder
- Career Advice
- Explore Majors
- Questions And Answers
- Interview Questions

Clinical Research Associate Vs Regulatory Affairs Specialist
The differences between clinical research associates and regulatory affairs specialists can be seen in a few details. Each job has different responsibilities and duties. While it typically takes 1-2 years to become a clinical research associate, becoming a regulatory affairs specialist takes usually requires 2-4 years. Additionally, a regulatory affairs specialist has an average salary of $70,060, which is higher than the $62,966 average annual salary of a clinical research associate.
The top three skills for a clinical research associate include patients, informed consent and CRA. The most important skills for a regulatory affairs specialist are regulatory affairs, FDA, and regulatory agencies.
Clinical research associate vs regulatory affairs specialist overview
| Clinical Research Associate | Regulatory Affairs Specialist | |
| Yearly Salary | ||
| Hourly rate | $30.27 | $33.68 |
| Growth Rate | - | |
| Number Of Jobs | ||
| Job Satisfaction | - | - |
| Most Common Degree | ||
| Average Age | ||
| Years Of Experience | 2 | 4 |
What does a Clinical Research Associate do?
A clinical research associate is responsible for assisting medical professionals in clinical trials and conducting research studies on medications and medical procedures. Clinical research associates monitor the research materials, ensuring its safety and reliability through trial procedures, writing comprehensive reports of results, and disseminating information across the concerned parties. They also provide recommendations on improving clinical processes, reiterating protocol requirements, and maintaining strict confidentiality of the trial subjects. A clinical research associate must have extensive knowledge of the medical industry, including its disciplines and principles, to perform duties accurately under minimal supervision.
What does a regulatory affairs specialist do?
A regulatory affairs specialist assists in securing and maintaining government approval for nutritional products, drugs, medical devices, and related supplies. They are often employed by medical, pharmaceutical, and biotechnology companies. They may also work in the government or law. Typically, they work on document preparation, file maintenance, information management, and coordination of tasks across various departments. They expanded their duties and responsibilities as a result of company acquisitions and restructuring, worldwide globalization of markets, and constantly evolving regulations.
Clinical research associate vs regulatory affairs specialist salary
Clinical research associates and regulatory affairs specialists have different pay scales, as shown below.
| Clinical Research Associate | Regulatory Affairs Specialist | |
| Average Salary | $62,966 | $70,060 |
| Salary Range | Between $43,000 And $91,000 | Between $49,000 And $99,000 |
| Highest Paying City | San Francisco, CA | Santa Rosa, CA |
| Highest Paying State | California | California |
| Best Paying Company | Meta | Meta |
| Best Paying Industry | Pharmaceutical | - |
Differences between clinical research associate and regulatory affairs specialist education
There are a few differences between a clinical research associate and a regulatory affairs specialist in terms of educational background:
| Clinical Research Associate | Regulatory Affairs Specialist | |
| Most Common Degree | Bachelor's Degree, 65% | Bachelor's Degree, 66% |
| Most Common Major | Biology | Business |
| Most Common College | University of Pennsylvania | Stanford University |
Clinical research associate vs regulatory affairs specialist demographics
Here are the differences between clinical research associates' and regulatory affairs specialists' demographics:
| Clinical Research Associate | Regulatory Affairs Specialist | |
| Average Age | 44 | 46 |
| Gender Ratio | Male, 28.3% Female, 71.7% | Male, 37.5% Female, 62.5% |
| Race Ratio | Black or African American, 2.5% Unknown, 7.4% Hispanic or Latino, 13.7% Asian, 13.0% White, 62.9% American Indian and Alaska Native, 0.5% | Black or African American, 10.7% Unknown, 4.5% Hispanic or Latino, 15.7% Asian, 8.2% White, 60.1% American Indian and Alaska Native, 0.8% |
| LGBT Percentage | 9% | 12% |
Differences between clinical research associate and regulatory affairs specialist duties and responsibilities
Clinical research associate example responsibilities..
- Manage, schedule and train up to 15 CRAs.
- Recruit patients, attain patient inform consent form, educate subjects on compliance, and ensure patient safety per ICH guidelines.
- Manage site TMF to ensure communication requirements adherence
- Manage CRO and regional monitor to complete close out activities, including device accountability management.
- Manage regional academic and community base oncology practices as the primary contact for all communications and support.
- Manage the monitoring CRO and the data clean-up efforts for a 510k submission and interim/annual study reports by effectively collaborating cross-functionally.
Regulatory Affairs Specialist Example Responsibilities.
- Gather, evaluating, organizing, managing and collating information in a variety of formats.
- Manage CRO (regulatory) in support of the company's approve product in Europe.
- Manage and submit electronic EPA product registrations.
- Manage and submit regulatory permit and notification applications to USDA to import, move and release regulate plant materials.
- Support complaint handling process and screening, ensure FDA 21 CFR compliance.
- Close complaints receive from external customers in compliance with FDA, ISO, QSR and other regulatory guidelines.
Clinical research associate vs regulatory affairs specialist skills
- Patients, 9%
- Informed Consent, 7%
- Clinical Trials, 6%
- Clinical Trial Management, 5%
- Oncology, 4%
- Regulatory Affairs, 10%
- Regulatory Agencies, 7%
- Medical Devices, 6%
- Regulatory Compliance, 5%
- Regulatory Submissions, 5%
Clinical Research Associate vs. Similar Jobs
- Clinical Research Associate vs Coordinator
- Clinical Research Associate vs Assistant
- Clinical Research Associate vs Clinical Trials Associate
- Clinical Research Associate vs Clinical Trial Manager
- Clinical Research Associate vs Project Manager
- Clinical Research Associate vs Medical Science Liaison
- Clinical Research Associate vs Data Analyst
- Clinical Research Associate vs Regulatory Affairs Specialist
- Clinical Research Associate vs Pharmacovigilance Safety Expert
- Clinical Research Associate vs Certified Medical Technician
- Clinical Research Associate vs Senior Clinical Research Associate
- Clinical Research Associate vs Research Nurse
- Clinical Research Associate vs Senior Research Associate
- Clinical Research Associate vs Research Project Coordinator
- Clinical Research Associate vs Study Coordinator
Clinical Research Associate Related Careers
- Clinical Associate
- Clinical Coordinator
- Clinical Project Manager
- Clinical Research Assistant
- Clinical Research Coordinator
- Clinical Research Manager
- Clinical Trial Coordinator
- Clinical Trial Manager
- Coordinator And Research Assistant
- Laboratory Manager
- Research Administrator
- Research Associate
- Research Coordinator
- Research Nurse
- Research Project Coordinator
Clinical Research Associate Related Jobs
- Clinical Associate Employment Near Me
- Clinical Coordinator Employment Near Me
- Clinical Project Manager Employment Near Me
- Clinical Research Assistant Employment Near Me
- Clinical Research Coordinator Employment Near Me
- Clinical Research Manager Employment Near Me
- Clinical Trial Coordinator Employment Near Me
- Clinical Trial Manager Employment Near Me
- Coordinator And Research Assistant Employment Near Me
- Laboratory Manager Employment Near Me
- Research Administrator Employment Near Me
- Research Associate Employment Near Me
- Research Coordinator Employment Near Me
- Research Nurse Employment Near Me
- Research Project Coordinator Employment Near Me
Clinical Research Associate Jobs By Location
- Clinical Research Associate Allen, TX
- Clinical Research Associate El Paso, TX
- Clinical Research Associate Forest Park, IL
- Clinical Research Associate Kent, OH
- Clinical Research Associate Lauderdale Lakes, FL
- Clinical Research Associate Long Beach, CA
- Clinical Research Associate Mount Pleasant, SC
- Clinical Research Associate Okemos, MI
- Clinical Research Associate Omaha, NE
- Clinical Research Associate Phoenix, AZ
- Clinical Research Associate Portland, OR
- Clinical Research Associate Rancho Cucamonga, CA
- Clinical Research Associate Redlands, CA
- Clinical Research Associate Somerville, MA
- Clinical Research Associate Washougal, WA
- Zippia Careers
- Executive Management Industry
- Clinical Research Associate
Browse executive management jobs
The Role of Regulatory Affairs in Clinical Research

Introduction to Regulatory Affairs in Clinical Research
Regulatory affairs play a pivotal role in ensuring the safety, efficacy, and quality of pharmaceutical products and medical devices.
In the field of clinical research, regulatory affairs professionals are at the forefront of ensuring compliance with the laws and regulations set forth by regulatory agencies such as the FDA (Food and Drug Administration), EMA (European Medicines Agency), MHRA (Medicines and Healthcare products Regulatory Agency), and PMDA (Pharmaceuticals and Medical Devices Agency). Their expertise is essential in navigating the complex landscape of clinical trials and ensuring that research studies meet the necessary regulatory requirements.
Importance of regulatory affairs in clinical research
The importance of regulatory affairs in clinical research cannot be overstated. These professionals are responsible for ensuring that clinical trials adhere to strict regulatory guidelines, which are in place to protect the rights and welfare of study participants. Regulatory affairs professionals work closely with researchers, sponsors, and regulatory agencies to ensure that all necessary approvals and documentation are in place before a clinical trial can begin. They are also responsible for monitoring ongoing trials to ensure compliance with regulatory requirements and for reporting any adverse events or safety concerns to the appropriate regulatory authorities.
Furthermore, regulatory affairs professionals play a crucial role in the post-approval phase of clinical research. They are responsible for submitting regulatory filings and maintaining compliance with regulatory requirements throughout the lifecycle of a product. This includes ensuring that any changes to the product, such as formulation updates or manufacturing process changes, are properly documented and approved by the regulatory authorities.
Without the expertise of regulatory affairs professionals, the entire process of bringing a new drug or medical device to market would be fraught with risk and uncertainty.
Roles and responsibilities of regulatory affairs professionals
Regulatory affairs professionals have a wide range of roles and responsibilities in clinical research. They are responsible for interpreting and implementing regulations, guidelines, and policies related to clinical trials. This includes staying up to date with the latest developments in regulatory requirements and ensuring that all stakeholders are aware of their obligations. They are also responsible for preparing and submitting regulatory submissions, including investigational new drug applications (INDs) and marketing authorization applications (MAAs), and for coordinating with regulatory agencies throughout the review and approval process.
In addition, regulatory affairs professionals are responsible for maintaining compliance with regulatory requirements throughout the lifecycle of a product. This includes ensuring that all documentation, such as clinical trial protocols, informed consent forms, and case report forms, are complete and accurate. They are also responsible for monitoring ongoing trials to ensure that they are conducted in accordance with the approved protocols and that any deviations or safety concerns are properly addressed.
Regulatory agencies in clinical research - FDA, EMA, MHRA, PMDA
Regulatory agencies play a crucial role in overseeing and regulating clinical research activities. These agencies are responsible for ensuring the safety and efficacy of drugs and medical interventions before they are approved for use in the general population. Some of the major regulatory agencies involved in clinical research include the Food and Drug Administration (FDA) in the United States, the European Medicines Agency (EMA) in Europe, the Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom, and the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan.
The FDA is perhaps the most well-known regulatory agency in the world. It is responsible for regulating drugs, biologics, medical devices, and food products in the United States. The FDA sets rigorous standards for clinical trial design, data collection, and analysis, and reviews all applications for new drug approvals. The agency also conducts inspections and audits to ensure compliance with regulatory requirements.
In Europe, the EMA is the regulatory agency responsible for the evaluation and supervision of medicinal products. It plays a key role in the approval process for new drugs and provides scientific advice to pharmaceutical companies. The EMA collaborates closely with national regulatory agencies in European Union member states to ensure consistent standards and regulatory oversight across the region.
The MHRA is the regulatory agency responsible for ensuring the safety, quality, and efficacy of medicines in the United Kingdom. It assesses the safety and regulatory compliance of clinical trials conducted in the UK and provides guidance and support to researchers and sponsors.
In Japan, the PMDA is the regulatory agency responsible for the evaluation and approval of pharmaceuticals and medical devices. It works closely with the Ministry of Health, Labour, and Welfare to ensure the safety and efficacy of drugs and medical interventions.
These regulatory agencies play a critical role in safeguarding the interests of study participants and the general public by ensuring that clinical trials are conducted in compliance with applicable regulations and guidelines. Their oversight and review processes are essential for the approval and commercialization of new drugs and medical interventions.
Regulatory requirements for clinical trials
Clinical trials are subject to a wide range of regulatory requirements to ensure the safety and well-being of study participants. These requirements vary depending on the country or region in which the trial is being conducted. In general, regulatory requirements for clinical trials include obtaining ethical approval from an institutional review board (IRB) or ethics committee, obtaining regulatory approval from the appropriate regulatory agency, and adhering to Good Clinical Practice (GCP) guidelines.
Ethical approval is obtained by submitting a detailed study protocol and informed consent form to an IRB or ethics committee. The IRB or ethics committee reviews the protocol to ensure that the study is scientifically valid and that the rights and welfare of study participants are protected. Regulatory approval is obtained by submitting a regulatory submission, such as an IND or MAA, to the appropriate regulatory agency. The regulatory agency reviews the submission to ensure that the study meets the necessary regulatory requirements.
Good Clinical Practice guidelines provide a set of internationally recognized standards for the design, conduct, recording, and reporting of clinical trials. These guidelines ensure that the data collected from clinical trials is reliable and can be used to support regulatory decisions. Compliance with GCP guidelines is essential for regulatory approval and for ensuring the integrity of the clinical trial data.
Regulatory submissions and approvals
Regulatory submissions and approvals are a critical part of the clinical research process. These submissions and approvals are necessary for initiating a clinical trial, obtaining regulatory clearance to market a new product, and maintaining compliance throughout the product lifecycle.
In the pre-approval phase, regulatory affairs professionals are responsible for preparing and submitting regulatory submissions to the appropriate regulatory agency. These submissions include detailed information about the study protocol, the investigational product, and the safety and efficacy data obtained from preclinical and clinical studies. The regulatory agency reviews the submission and may request additional information or clarification before granting approval to proceed with the clinical trial.
In the post-approval phase, regulatory affairs professionals are responsible for maintaining compliance with regulatory requirements and for submitting post-marketing reports and updates to the regulatory agency. These reports and updates may include information about adverse events, changes to the product labeling, and updates to the manufacturing process. Regulatory affairs professionals must ensure that all changes are properly documented and approved by the regulatory agency to maintain compliance and ensure patient safety.
Challenges in regulatory affairs in clinical research
Regulatory affairs professionals in clinical research face numerous challenges in their work. These challenges arise from the constantly evolving regulatory landscape, the complexity of the drug development process, and the need to balance regulatory requirements with efficient and timely completion of clinical trials.
Some of the key challenges faced by regulatory affairs professionals include the following:
1. Evolving regulations and guidelines: Regulatory requirements are constantly evolving as new scientific discoveries are made and as regulators strive to ensure the safety and efficacy of drugs and medical interventions. Regulatory affairs professionals must stay up-to-date with these changes and ensure that clinical trials and studies are conducted in compliance with the latest regulations and guidelines.
2. Global harmonization: Clinical trials are increasingly conducted on a global scale, with multiple sites in different countries participating in a single trial. This presents challenges in terms of harmonizing regulatory requirements across different jurisdictions and ensuring consistent standards of ethical conduct and data integrity.
3. Time and resource constraints: Clinical trials are often time-sensitive, with strict deadlines for recruitment, data collection, and reporting. Regulatory affairs professionals must work within these tight timelines while ensuring compliance with regulatory requirements. This can be particularly challenging when dealing with complex or large-scale trials.
4. Communication and collaboration: Regulatory affairs professionals play a crucial role in facilitating communication and collaboration between researchers, sponsors, and regulatory agencies. Effective communication is essential for the successful completion of clinical trials and the timely approval of new drugs. However, communication breakdowns or delays can result in regulatory delays or non-compliance issues.
5. Regulatory inspections and audits: Regulatory agencies conduct inspections and audits to assess compliance with regulatory requirements and to ensure the integrity and reliability of trial data. These inspections can be stressful and time-consuming for both researchers and regulatory affairs professionals, requiring meticulous preparation and attention to detail.
6. Adapting to technological advancements: The use of technology in clinical research is rapidly evolving, with new tools and platforms being developed to streamline data collection, analysis, and reporting. Regulatory affairs professionals must stay abreast of these technological advancements and ensure that they are implemented in compliance with regulatory requirements.
Despite these challenges, regulatory affairs professionals play a crucial role in ensuring the successful completion of clinical trials and the approval of new drugs. Their expertise and attention to detail contribute to the integrity and reliability of the data generated and the safety and well-being of study participants.
Career opportunities in regulatory affairs
The field of regulatory affairs offers a wide range of career opportunities for professionals with a passion for healthcare and a strong attention to detail. Regulatory affairs professionals can work in a variety of settings, including pharmaceutical companies, medical device manufacturers, contract research organizations (CROs), and regulatory consulting firms.
Entry-level positions in regulatory affairs typically require a bachelor's degree in a scientific or healthcare-related field, along with a strong understanding of regulatory requirements and guidelines. As professionals gain experience and expertise, they can advance to more senior positions, such as regulatory affairs manager or director. These positions typically require a master's degree or higher, along with extensive experience in regulatory affairs and a deep understanding of the regulatory landscape.
Regulatory affairs professionals can also specialize in specific areas, such as clinical trials, post-marketing surveillance, or regulatory strategy. Specialization can enhance career prospects and provide opportunities for advancement and leadership within the field.
To pursue a career in regulatory affairs, individuals typically need a strong background in life sciences or a related field. A bachelor's or master's degree in pharmacy, medicine, biochemistry, or a similar discipline is often required. In addition, obtaining certifications from professional organizations such as the Regulatory Affairs Professionals Society (RAPS) or the Association of Clinical Research Professionals (ACRP) can enhance career prospects and demonstrate expertise in regulatory affairs.
In conclusion, regulatory affairs play a crucial role in ensuring the safety, efficacy, and quality of pharmaceutical products and medical devices in clinical research. Regulatory affairs professionals are responsible for navigating the complex regulatory landscape, ensuring compliance with regulatory requirements, and maintaining patient safety throughout the product lifecycle.
The field of regulatory affairs offers numerous career opportunities for professionals with a passion for healthcare and a strong attention to detail. By working closely with regulatory agencies, researchers, and sponsors, regulatory affairs professionals contribute to the advancement of medical knowledge and the improvement of patient outcomes.
Sign up for post alerts
Icon & you the potential of together..
Careers that improve the lives of patients, our clients and each other. Are you ready to make a difference?
Related jobs at ICON
India, Chennai
Clinical Trial Management
Remote Working
Office Based
Business Area
ICON Full Service & Corporate Support
Job Categories
Regulatory Document Management
Description
At ICON, it’s our people that set us apart. Our diverse teams enable us to become a better partner to our customers and help us to fulfil our mission to advance and improve patients’ lives. Our ‘Own I
Expiry date

UK, Reading
Hybrid: Office/Remote
Drug / Device Regulatory Affairs
Director, Regulatory Affairs (Clinical and Medical Device Regulatory Submissions)Home or office based, EU wideThe Role:Actively anticipate, develop and implement initial or alternative regulatory stra

Mexico, Mexico City
Mexico City

United States
Regulatory, Drug Safety/ Quality Assurance & Other roles
ICON Strategic Solutions
Regulatory Affairs
As a Director, Regulatory Affairs, you will be joining the world’s largest & most comprehensive clinical research organisation, powered by healthcare intelligence.
2024-112716

Related stories
.png)
Teaser label
Content type
Publish date
Unlock Your Potential: Discover Your Ideal Career In today's rapidly evolving job market, finding the right career path can be a daunting task. With a myriad of options available, it can be challe
Explore how career aptitude tests can help you uncover your strengths, interests, and values to find the perfect career fit.
.png)
Insights from Interns: Real Experiences at ICON's Swansea Office Internships offer a unique chance to bridge the gap between academic studies and the professional world. At ICON's Swansea office,
Curious about what an internship at ICON’s Swansea office is like? Hear from three interns as they share their experiences.

Immunisation research plays a crucial role in global health, with clinical trials forming the backbone of vaccine development and improvement. In our latest interview, we delve into the world of in
Discover insights from Megan, Senior Clinical Trial Manager at ICON, on global influenza vaccine research.
Recently viewed jobs
A better career. A better world. A better you.
Browse popular job categories below or search all jobs above
- [email protected]
- +1 847-454-3872

The Role of Regulatory Affairs in Clinical Research

- July 08, 2024
Clinical research is a complex and highly regulated field that ensures the safety, efficacy, and ethical standards of new medical treatments and interventions. At the heart of this intricate process lies the role of regulatory affairs. Regulatory affairs professionals are pivotal in navigating the maze of regulations and guidelines that govern clinical research. In this blog, we delve into the essential role of regulatory affairs in clinical research, exploring their responsibilities, challenges, and the impact they have on the development of new therapies.
Understanding Regulatory Affairs in Clinical Research
Regulatory affairs encompass the process of ensuring that clinical research and the resulting medical products comply with all applicable laws, regulations, and guidelines. This field bridges the gap between regulatory authorities and the various stakeholders involved in clinical research, including pharmaceutical companies, researchers, and healthcare providers. Regulatory affairs professionals are responsible for preparing, submitting, and managing documentation required for regulatory approvals, as well as maintaining compliance throughout the lifecycle of a clinical trial and beyond.
Key Responsibilities of Regulatory Affairs Professionals
- Regulatory Strategy Development One of the primary roles of regulatory affairs professionals is to develop and implement regulatory strategies that align with the goals of the clinical trial and the requirements of regulatory authorities. This involves understanding the regulatory landscape, anticipating potential challenges, and devising plans to address them effectively.
- Preparing Regulatory Submissions Regulatory affairs professionals prepare a variety of documents required for regulatory submissions, including Investigational New Drug (IND) applications, Clinical Trial Applications (CTAs), and New Drug Applications (NDAs). These documents provide comprehensive information about the trial design, protocols, patient safety measures, and scientific rationale.
- Ensuring Compliance with Regulations Compliance with local and international regulations is crucial in clinical research. Regulatory affairs professionals ensure that all aspects of the clinical trial, from patient recruitment to data collection and reporting, adhere to the relevant regulatory requirements. This involves continuous monitoring and auditing to identify and rectify any compliance issues.
- Liaising with Regulatory Authorities Regulatory affairs professionals serve as the primary point of contact between the clinical research team and regulatory authorities. They facilitate communication, address queries, and provide additional information or clarification as needed. This liaison role is critical in ensuring a smooth and efficient regulatory review process.
- Managing Regulatory Documentation Maintaining accurate and up-to-date regulatory documentation is essential for compliance and successful submissions. Regulatory affairs professionals manage extensive documentation, including trial master files, safety reports, and correspondence with regulatory authorities. Proper documentation management ensures transparency and accountability throughout the clinical trial process.
The Impact of Regulatory Affairs on Clinical Research
Regulatory affairs play a pivotal role in the successful execution of clinical trials and the development of new medical treatments. Their impact is far-reaching and encompasses various aspects of clinical research:
- Ensuring Patient Safety and Ethical Conduct Regulatory affairs professionals are instrumental in ensuring that clinical trials adhere to the highest standards of patient safety and ethical conduct. They ensure that trials are designed and conducted in a manner that minimizes risks and protects the rights and well-being of participants.
- Facilitating Market Access Successful regulatory submissions and approvals are critical for bringing new therapies to market. Regulatory affairs professionals work diligently to ensure that clinical trials generate robust data that meets regulatory requirements, facilitating timely market access for new treatments.
- Enhancing Scientific Rigor By ensuring compliance with regulatory standards, regulatory affairs professionals contribute to the scientific rigor and credibility of clinical research. Their efforts ensure that trial results are reliable, reproducible, and can withstand regulatory scrutiny.
- Supporting Innovation Regulatory affairs professionals play a crucial role in supporting innovation in clinical research. They work with researchers and developers to navigate regulatory pathways, identify opportunities for expedited approvals, and bring innovative therapies to patients faster.
Conclusion
The role of regulatory affairs in clinical research is indispensable. Regulatory affairs professionals ensure that clinical trials are conducted ethically, safely, and in compliance with all relevant regulations. Their work is critical to the development of new medical treatments and the advancement of medical science. As the regulatory landscape continues to evolve, the expertise and dedication of regulatory affairs professionals will remain essential in navigating the complexities of clinical research and bringing new therapies to patients.
By bridging the gap between regulatory authorities and clinical researchers, regulatory affairs professionals play a vital role in shaping the future of healthcare. Their contributions ensure that clinical trials uphold the highest standards of safety, efficacy, and ethical conduct, ultimately benefiting patients and advancing medical innovation.
Recent Posts

The Vital Role of Workforce Diversity and Inclusion
- June 06, 2024

Diversity in Clinical Research Workforce
- September 22, 2022
Question: What are the differences between clinical research administration and regulatory affairs? Clinical Research Administration vs. Regulatory Affairs
Updated: november 30, 2022.
Answer: Clinical research administration and regulatory affairs are two related jobs that are integral to the rigorous process of bringing pharmaceuticals and medical devices from the discovery phase to market. Clinical research administration involves direct oversight of clinical trials that test the safety and efficacy of new medications, biologics, and devices. Ensuring that those clinical trials adhere to applicable federal and international laws and statutes is the purview of regulatory affairs.
Prior to approval by the Food and Drug Administration (FDA), the European Medicines Agency (EMA), and other affiliates of the International Coalition of Medicines Regulatory Authorities (ICMRA), pharmaceuticals and medical devices must undergo formal clinical trials to demonstrate their safety and efficacy. Clinical research administrators work with research scientists and medical professionals to design, implement, and manage these clinical trials. This commonly involves setting up and managing clinical sites (e.g., research hospitals, medical centers, cancer centers…), enrolling test subjects, assigning human and material resources, securing and storing samples and data, documenting results, communicating with the FDA and internal review boards (IRBs), and more.
Regulatory affairs specialists are tasked with advising clinical research professionals and others overseeing clinical trials on adherence to the laws and regulations that govern the testing and approval of pharmaceuticals and medical devices. They serve as intermediaries between the FDA and the organization conducting the clinical trials, ensuring that all aspects of the testing process, from experimental design and sample size to implementation and analysis, comply with federal regulations. Whereas the responsibilities of clinical research administrators generally ends when the clinical trials conclude, regulatory affairs professionals are tasked with post-trials compliance in areas related to packaging, advertising, and marketing.
Thus, clinical research administrators and regulatory affairs specialists are crucial to facilitating the process by which new medical treatments and devices move from the development phase to approval. They each have distinct, yet complementary roles to play in ensuring safety, efficacy, and legality of pharmaceuticals, biologics, and medical devices.
Clinical Research Administration vs. Regulatory Affairs
One way to contrast the roles of clinical research and regulatory affairs professionals is through their relative proximity to actual clinical trials. While both are involved in the process, clinical research administrators typically fulfill their roles by engaging directly with the implementation and management of clinical trials, from setting up sites and enrolling patients to collecting patient samples. In contrast, regulatory affairs operates at a greater distance from the trials, scrutinizing protocols, standard operating procedures (SOPs), and processes to ensure all aspects of a clinical trial align with the most up-to-date regulations.
As a result, the focus of training in clinical trial administration differs from that in regulatory affairs. Understanding the foundational principles, scientific practices, and ethical standards for clinical trials is important in both fields. However, clinical trial administration combines managerial skills with knowledge of experimental design and scientific methodology, which includes familiarity with relevant regulations. Regulatory affairs, in contrast, focuses on the intricacies of the laws that clinical trials are subject to and how those laws are enforced.
There are thus areas of overlap and divergence in the training for these two professions. The table below illustrates some of the differences between clinical research administration and regulatory affairs using the types of courses that are commonly part of graduate curricula in these fields.
| Clinical Research Administration | Regulatory Affairs |
|---|---|
| Clinical Research Methods | Protection of Human Subjects |
| Clinical Trials Planning and Implementation | Food and Drug Law |
| Critical Evaluation of the Medical Literature | Ethical Concerns in Drug Discovery |
| Biostatistics | Quality Control and Safety in Clinical Trials |
| Data Management and Analysis | Post-Market Drug Safety and Marketing |
It is important to note that the management of clinical trials and regulatory compliance are closely linked and have a common purpose — to safely test the efficacy of pharmaceuticals, biologics, and medical devices and pilot them to market. Indeed, training to become a clinical research administrator typically involves learning about regulatory compliance, and training to become a regulatory affairs specialist includes cultivating a clear knowledge of how clinical trials are conducted.
For more information on clinical research and regulatory affairs, check out our Graduate Certificate Programs in Clinical Research Management and Graduate Certificate Programs in Regulatory Affairs and Regulatory Science pages.
Healthcare FAQs:
Healthcare graduate certificate programs.
- Graduate Certificate in Bioinformatics
- Graduate Certificate in Clinical Research
- Graduate Certificate in Gerontology
- Graduate Certificate in Global Health
- Graduate Certificate in Health and Wellness
- Graduate Certificate in Health Informatics
- Graduate Certificate in Healthcare Management and Administration
- Graduate Certificate in Nutrition
- Graduate Certificate in Patient Safety and Quality
- Graduate Certificate in Public Health
- Graduate Certificate in Regulatory Affairs
Voices Blog
Sciences, Mathematics and Biotechnology

Understanding Regulatory Affairs’ Impact on Clinical Trials
Certificate graduate, clinical trials manager jennifer cuvin better understands the interplay between both departments.
Medicinal products. Pharmaceuticals. Veterinary medicines. Medical devices. Food supplements.
Each of these products must go through strict regulations before hitting the market—and even afterward. Involved in all stages of drug development—from conception to clinical trial to approval and marketing—are highly skilled and trained regulatory affairs staff. Throughout the entire process, these professionals often work in teams, which include clinical researchers who manage the crucial clinical trial stages. Regulatory affairs specialists must understand the roles and responsibilities of clinical researchers and vice versa.
This continuous flow of information and understanding are what brought Jennifer Cuvin to our Professional Program in Regulatory Affairs .
"Because clinical operations work alongside regulatory associates,” Jennifer says, “I wanted to gain a better understanding of regulatory’s role and responsibilities. I wanted to be able to continue performing my current work duties without having to take much time off to explore other job functions.”
Deep Roots in Clinical Trials
Jennifer is no stranger to working alongside regulatory associates—she has 11 years of clinical research experience under her belt:
- More than six years working at Parexel , a clinical research organization, where she started as a clinical research associate assisting clinical research coordinators to ensure clinical trials were performed according to protocol. She also worked closely with sponsors as a clinical research coordinator, performing study procedures with patients and managing trials to completion of required data. From that role she moved into a project quality lead and then senior project quality lead, where she provided study support to clinical operations during the conduct of the study with CAPA consultation and support until resolution.
“I implemented risk-based quality interventions, verifying compliance with standard operating procedures and guidelines and protocol requirements, and also provided audit and inspection support.”
- Most recently, Jennifer has been working at Gilead for five years at the sponsor level in a clinical trial manager role. In this position, she manages studies from protocol development to completion of Clinical Study Reports in early phase clinical studies.
I learned something new in each class and was able to either use what I learned in my current role or have a better understanding of the process behind the requirements requested from my study team.
The Circle of Communications
It’s easy to see that during her professional career, Jennifer has had multiple interactions with regulatory affairs specialists. In order to do her job better, Jennifer wanted to make sure that she knew the ins and outs of regulatory affairs to ensure that communications between the two departments were as seamless as possible.
“I learned something new in each class and was able to either use what I learned in my current role or have a better understanding of the process behind the requirements requested from my study team,” Jennifer explains. “Knowing the responsibilities of regulatory—like the importance of the end of Phase-2 meetings—helped me understand what stage the drug compound was in during development. It helped with the overall big picture of drug development and the process that needs to happen before getting it to market.”
Seeing familiar faces as you continue to take the classes made for good networking relationships.
Not only did the coursework bring to life the details of regulatory affairs work, learning alongside a varied group of classmates helped her dig deeper into the coursework.
“It was nice to work with classmates and instructors with different backgrounds in different career fields with the same interest,” she says. “Seeing familiar faces as you continue to take the classes made for good networking relationships.”
Bringing Study Topics to Work
Ever the lifelong learner, the February 2018 certificate graduate is not only progressing in her own clinical research career, but she also has a deeper understanding of how her work impacts the regulatory affairs teams and her role in the drug-development process.
For example, Jennifer is working on two to four projects simultaneously (each at a different stage—be it initiation, maintenance or closure) from different therapeutic areas, such as liver disease, inflammation or oncology—all in the early phase department.
“I continue to manage trials from development to protocol, managing site conduct of clinical research units and contract monitoring through study completion and finalization of the clinical study report,” Jennifer explains.
Of having completed the professional program, Jennifer says, “It has helped me understand the relationship between our two working departments and the importance of collaboration.”
Stay up to date with courses and trends in Regulatory Affairs
Deepen your skills, regulatory affairs, related posts.

September 10 Online Event: Careers in Biotech

A Vision for Providing Optometric Health Care

Moving to the Other Side of the Glass
View the discussion thread.
- REQUEST INFO
MSHS in Regulatory Affairs and Clinical Research Leadership

Explore core competencies across two exciting concentrations
100% Online
24 Months*
Offered through The George Washington University (GW) School of Medicine and Health Sciences (SMHS), the online Master of Science in Health Sciences (MSHS) in Regulatory Affairs and Clinical Research Leadership program is designed to give you practical knowledge about the key elements of clinical research administration and regulatory affairs from a global perspective.
This program incorporates global regulatory strategy and clinical research industry-specific concepts across the curriculum to equip you with the critical thinking skills you need to succeed in these fields.
* The total number of credits and duration of the program depend on the number of transferred credits
To learn more about our programs, you can register for our upcoming events .
* Indicates required field
By providing your phone number on this request information form, you have authorized the George Washington University, and its representatives, to send you SMS/Text messages in conjunction with the services you have requested. Message and data rates may apply.
If you no longer wish to receive SMS/Text communications from GW SMHS, you will have the option to opt-out.
By submitting this form, you confirm you have read the Privacy Notice .
Accreditation and Rankings
- GW is accredited by the Middle States Commission on Higher Education
- #62 Best National University*
- #13 Best Online Bachelor's Programs*
- #7 Best Online Programs for Veterans*
* The U.S. News & World Report – 2024 Rankings

Program Highlights
- 100% online, no on-campus residency required
- Complete your degree in 24 months part-time
- 2 concentration options: regulatory affairs and clinical research administration
- Developed in collaboration with regulatory affairs and clinical research professionals working in the industry and governmental agencies including the FDA and NIH
- Strong national alumni network
Through this 36-credit program, you’ll have the option of choosing between two unique concentrations: regulatory affairs and clinical research administration.
The courses address concepts in regulatory strategy and guidance, investigational product development and the business, managerial, safety, scientific and ethical components of clinical research.
VIEW COURSES
Who Is the Ideal Student for This Program?
The online MSHS in Regulatory Affairs and Clinical Research Leadership program is ideal for regulatory affairs, clinical research and health care professionals looking to lead regulatory strategy and therapeutic product development in the evolving field of health care.
Program Outcomes
As a graduate of this program, you will be fully prepared to:
- Create clinical and regulatory plans for the development of investigational therapeutics that adhere to domestic and international laws, regulations, and pre- and post-approval requirements
- Lead interdisciplinary team to develop strategies to ensure successful pharmaceutical/medical device product development, regulatory approvals, and marketing activities
- Formulate strategies to ensure clinical trial diversity, ethical conduct, patient safety, data integrity, and compliance with domestic and international laws and regulations when developing new therapeutics
- Strategize the therapeutic product lifecycle to address the evolving global legal, clinical, and regulatory requirements in the healthcare industry
Career Outlook
The online MSHS in Regulatory Affairs and Clinical Research Leadership program takes a real-world approach to create effective leaders and communicators across a range of positions.
According to the U.S. Department of Labor and the National Center for O*NET Development, the median wage for Regulatory Affairs Managers is $128,620 annual, with 94,400 projected job openings by 2032. 1 In addition, clinical research director positions have an average annual salary of $121,579. 2
Meet the growing demand for qualified professionals while you prepare to fill a variety of roles, including:
- Regulatory Affairs Specialist: $73,090 avg. salary 3
- Regulatory Affairs Manager: $110,916 avg. salary 4
- Senior Chemistry, Manufacturing, and Controls (CMC) Manager: $115,000 avg. salary 5
- Clinical Research Coordinator, $52,319 avg. salary 6
- Clinical Research Associate, $73,307 avg. salary 7
- Clinical Data Manager, $78,920 avg. salary 8
Admission Requirements
To be accepted into this program, you must have:
- Completed application
- 3.0 GPA or above on a 4.0 scale
- A bachelor’s degree from a regionally accredited institution
- Official transcripts from every college and university attended*
- Personal statement
- Two letters of recommendation from a current/former employer or instructor
- Previous work experience: health care related experience preferred
*All non-U.S. transcripts (including those in English) must be evaluated by an accredited foreign credential agency.
SEE ALL REQUIREMENTS
Tuition Details
The MSHS in Regulatory Affairs and Clinical Research Leadership program at GW consists of 36 credit hours. Please find the cost per credit hour and total estimated program costs here .
Note: Tuition rates are subject to change and additional fees may vary by program.
GET TUITION DETAILS
Meet the Program Directors

GW’s experienced faculty provide you with the rich, practical knowledge and support needed for you to succeed in the program and in your career.

Kathy Thoma, EdD, CCRP, CPH
Dr. Thoma is the Program Director for the BSHS in Clinical Research Administration Program, the Dual Degree Clinical Research Administration Programs, and the Graduate Certificate in Clinical Research Administration Program. She is also the Assistant Program Director for the MSHS in Regulatory Affairs and Clinical Research Leadership Program, and an Assistant Professor in the Department of Clinical Research and Leadership. She has over 20 years of experience in clinical research, health services research and educational research.
Before coming to the George Washington University School of Medicine and Health Sciences, she was the director of research and a clinical research specialist at the University of Florida Center for HIV/AIDS Research, Education and Service (UF CARES) where she managed many NIH and industry-sponsored clinical trials during her tenure. Her particular expertise involved working with NIH-sponsored trials through the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network and the Pediatric HIV/AIDS Cohort Study (PHACS) network. She has also held positions as research project manager for the Pediatric Research in Office Settings (PROS) Network at the American Academy of Pediatrics, director of educational research at Florida State College at Jacksonville, and research project coordinator at the University of Illinois at Chicago’s College of Medicine/School of Public Health. She earned a doctorate in Educational Leadership with a cognate in Public Health from the University of North Florida.
She is a Certified Clinical Research Professional (CCRP) through the Society of Clinical Research Associates (SOCRA) and holds the Certified in Public Health (CPH) credential from the National Board of Public Health Examiners.
Her research interests include patient engagement and centricity in clinical trials, increasing diversity and inclusion in clinical trials, health literacy, health disparities and the social determinants of health, health equity, and distance education in the health sciences.
- 11-9199.01 – Regulatory Affairs Managers. (2023). Retrieved October 24, 2023, from Onetonline.org
- Payscale.com (2023 October 11). Clinical Research Director. Retrieved October 24 2023, from Payscale - Clinical Research Director Salary
- Payscale (2023 October 8). Regulatory Affairs Specialist. Retrieved October 24, 2023, from Payscale - Regulatory Affairs Specialist Salary
- Payscale (2023 October 4). Regulatory Affairs Manager. Retrieved October 24, 2023, from Payscale - Regulatory Affairs Manager Salary
- Payscale (2023 January 8) Senior Chemistry and Manufacturing Controls (CMC) Project Manager. Retrieved October 24, 2023, from Payscale - Senior CMC Project Manager Salary
- Payscale.com (2023, October 10). Clinical Research Coordinator. Retrieved October 24, 2023, from Payscale - Clinical Research Coordinator Salary
- Payscale.com (2023, October 9). Clinical Research Associate. Retrieved October 24, 2023, from Payscale - Clinical Research Associate Salary
- Payscale.com (2023, October 10). Clinical Data Manager. Retrieved October 24, 2023, from Payscale - Clinical Data Manager Salary
Curriculum Details
36 TOTAL CREDITS REQUIRED
The online MSHS in Regulatory Affairs and Clinical Research Leadership program consists of seven core courses, four concentration course, and one elective course for a total of 36 credits.
This program is 100% online, features no on-campus residencies, and you can graduate in just 24 months part-time.
Core Courses
Foundation of regulatory affairs and clinical research in therapeutic development in U.S., EU and Japan. Roles in developing products, navigating the regulatory review and approval process, and maintaining products on the market.
Overview of therapeutic development through the analyses of the critical elements of the product lifecycle, assessment of non-clinical and clinical data, and integration of strategic business needs and post-marketing efforts in planning regulatory strategy. Prerequisite: RCR 6201.
This course explores international regulatory requirements for the development and approval of new pharmaceutical products around the world. Prerequisite: RCR 6201.
Students explore the basic concepts of epidemiology which includes various epidemiological study designs used to examine disease frequency, cause-effect relationships between risk factors and disease states, and effects of bias as examples. Students apply epidemiologic concepts in the context of translational research.
Overview of principles related to leadership, including theories and styles, organizational management and values, communication strategies, and change in the context of healthcare systems.
Overview of business principles related to health care systems and leadership, focusing on strategic management of health care service delivery in various settings. Credit cannot be earned for this course and HSCI 6241.
Core Courses – Choose one of the following based on concentration
Theories of leadership and change are integrated in the development of change proposals for the regulatory affairs field. The development of leadership solutions to problems in leading regulatory strategic change; integration of all field coursework into implementation plans for health care system changes.
A capstone course focusing on the concept of leadership within the contexts of health professionals, health systems, and health policy.
RAFF Concentration Courses
Development and evaluation of the regulatory affairs strategies that support device and diagnostics development. Research science, study design, master file, risk/benefit analyses, product specifications and milestone identification, IDE, 510K, PMA. Prerequisites: RCR 6201
The planning and conduct of clinical trials. Topics include protocol development, study design, post-marketing surveillance, and evaluation and assessment of regulatory submissions. Strategies for achieving clinical development goals. Prerequisite: RCR 6201.
Analysis and evaluation of regulatory affairs compliance strategies and guidelines. Pre and post marketing compliance of medical products, oversight, labeling, advertising and use. Prerequisites: RCR 6201
Exploration of FDA-regulated advertising and promotion of pharmaceutical drugs. Focus on pre- and post-market issues for prescription drugs and management of risks and compliance surrounding medical and commercial communications. Prerequisites: RCR 6201 and RCR 6202.
CRA Concentration Courses
This course explores regulatory, policy, ethical and practical considerations associated with the engagement, recruitment, retention and interaction with human research subjects.
Integration of project management principles, decision making models, cross-cultural competency, and interdisciplinary team dynamics to facilitate effective and efficient conduct of clinical trials.
This course explores how to manage risk and safety assessments to ensure quality in clinical research.
Key stakeholder roles, responsibilities, and processes associated with monitoring, auditing, and oversight in clinical trial conduct.
RAFF Concentration Electives (Take One)
Basic concepts and methods of biostatistics applied to translational research. Topics include distributions, populations and sample selection, variables, interaction and confounding, hypothesis formulation, correlation, t-tests, ANOVA, regression, and chi.
CRA Concentration Electives (Take One)
Application of leadership and organizational change theories and principles to the implementation of quality and patient safety initiatives. Focus on strategies for developing the culture and infrastructure needed to support patient safety and continuous quality improvement.
NOTE: All classes are three credits each.
|
|
|
| |
| March 20, 2025
| July 20, 2024
| December 1, 2024
|
Admissions Requirements
To be accepted to the MSHS in Regulatory Affairs and Clinical Research Leadership program (100% online), you must have:
- Personal statement: Applicants must include a 250–500 word essay describing your reasons for undertaking study at GW, your academic objectives, career goals, and related qualifications including collegiate, professional, and community activities relevant to your program of interest. Include any substantial accomplishments not already mentioned on the application form.
- Two letters of recommendation from a current/former employer or instructor.
- Academic instructors who can strongly attest to your academic ability, and/or
- Individuals who served in a supervisory capacity for you, and who can strongly attest to your work ethic.
- The recommender cannot be from a family or friend.
- Recommender will submit a letter on letterhead with a signature and credentials.contact information via the application portal.
- Application fee: A non-refundable application fee of $80 is required. The application fee is waived for active-duty U.S. military, current GW students, degree-holding GW alumni, current McNair Program Scholars, and graduates of minority-serving institutions (MSI).
- Official transcripts from every college and university attended. All non-U.S. transcripts (including those in English) must be evaluated by an accredited foreign credential agency. Please find the list of member organizations here: https://www.naces.org/members .
International Students
International students should check with individual programs regarding eligibility for visa sponsorship. Generally, online and hybrid programs are not eligible for student visa sponsorship from GW. This would include transfer students from any other institution with an existing visa.
Official transcripts from institutions outside the U.S. must be accompanied by an official transcript evaluation from an accredited independent evaluating agency. Please be sure you request a detailed evaluation that includes all course titles, credit hours, grades, U.S. degree equivalency, grade-point averages (GPA), and date of degree conferral. For a list of acceptable foreign credential evaluation services, please visit NACES .
Applicants who are not U.S. citizens are also required to submit official test scores for the Test of English as a Foreign Language (TOEFL) or Pearson’s Test of English (PTE) Academics or the academic International English Language Test System (IELTS). To be considered for admission, there are required scores that you will need to meet. Score requirements may differ by school and program so check the admissions requirements for your program .
Supporting Documents and Official Transcript
Documents and Official Transcripts not submitted online should be mailed to:
Mail: George Washington University ATTN: Transcript Processing Center 1415 W 22 nd St. Suite 220 Oak Brook, IL 60523
Alternatively, official electronic transcripts can be sent to: [email protected]
As you explore our programs at George Washington University, our dedicated staff is here to support you. If you have any questions or want to know more, click the "Request More Information" button below, or email [email protected] .
Request More Information
The Common App is Open. Class of 2029, Apply Today!
- 30 Clinical Research and Regulatory Affairs Skills You Need to Advance Your Career
Seven Behind the Scenes Medical Jobs With No Patient Contact
- Seven Regulatory Affairs Jobs in Boston and How Regis Prepares You
- 10 Clinical Research Jobs in the Greater Boston Area
- Is a Master's in Regulatory Affairs and Clinical Research Worth It?
- Should I Get a Master's Degree or Certificate in Regulatory Affairs and Clinical Research?
- How To Get a Regulatory Affairs and Clinical Research Certificate and What You Can Do With It?
- Their Choice was Consistent: Physician Assistant
- The Nuclear Option
- Compassion, Ethics, and Values. All in a Day’s Work for a Social Worker
- Master's in Counseling vs Master's in Social Work
- Master's in Counseling Salary and Career Outlook
- MA in Counseling vs MS in Counseling: What's the Difference?
- What Can You Do With a Master's Degree in Counseling?
- How to Become a Licensed Counselor in Massachusetts
- What Can You Do With a Master's Degree in ABA?
- ABA vs Counseling: Which Master's Degree is Right for You?
- How to Become a Board Certified Behavior Analyst (BCBA): Five Steps
- Applied Behavior Analysis: What It is and How It Works
- How to Become a Counselor: Six Key Steps
- What Can You Do With a Bachelor's Degree in Psychology?
- Seven Top Public Health Careers in the Merrimack Valley
- Five Health Sciences Careers (and What They Pay) in the Merrimack Valley
- What Can You Do With a Health Sciences Degree?
- Careers for Veterans
- College Advice
- Completing Your Degree
- Dental Hygiene
- Marketing and Communications
- Medical Imaging
- Occupational Therapy
- Online Learning
- Public Health
- Speech-Language Pathology
The modern regulatory landscape is continuously evolving—both at the state and federal level. Professionals who work in regulatory affairs or clinical research are likely already aware of the benefits of working in behind the scenes medical jobs like this one. However, if you're considering advancing your career to a regulatory affairs role without prior experience, it's important to understand the requirements of working in this industry.
Clinical research and regulatory affairs professionals require a comprehensive skill set, as well as a thorough educational background. Here's an overview of the skills you'll need to obtain in clinical research and regulatory affairs, how to develop these skills, and how you can jumpstart your career.
Want to learn more about working in Regulatory and Clinical Research Management? Download Our Free Guide!

30 Clinical Research and Regulatory Affairs Skills
Working in clinical research and regulatory affairs requires an extensive list of skills. This includes hard skills (job specific proficiencies) and soft skills (interpersonal competencies that can easily transfer from one industry to another).
Hard Skills
According to our analysis of job postings data, the top hard skills required for clinical research and regulatory affairs professionals include:
- Pharmaceuticals
- Clinical trials
- Regulatory affairs
- Project management
- Clinical research
- Regulatory compliance
- Drug development
- Pre-clinical development

Obtaining the necessary hard skills for a career in clinical research or regulatory affairs isn’t always easy. It typically requires prior working experience or advanced education in a clinical research or regulatory affairs program. If you're hoping to acquire these skills, it's important to find opportunities to obtain hands-on experience with each.
For example, if you're considering pursuing advanced education, make sure your program includes internship or co-op opportunities. These experiences not only provide valuable real-world exposure, but also offer networking opportunities to enhance your career prospects.
Soft Skills
As you progress toward more management-related roles in your career, soft skills become increasingly important. Effective leadership and the ability to keep a team motivated are crucial when overseeing a team.
According to our analysis of job postings data, the top soft skills required for these professionals include:
- Communications
- Detail oriented
- Problem solving
- Interpersonal communications

However, finding a program that emphasizes management skills as well as job-specific responsibilities can go a long way in fostering your soft and hard skills.
Software Skills
In addition to the hard and interpersonal skills required for regulatory affairs and clinical research professionals, it's also important to familiarize yourself with the software skills needed to advance your career in this field.
According to our analysis of job postings data, the top software skills required for these professionals include:
- Microsoft Office
- Microsoft Excel
- Microsoft PowerPoint
- Microsoft Outlook
- R (Programming language)
- Microsoft Word
- Python (Programming language)
- Microsoft Access
- Electronic Data Capture (EDC)
- Microsoft SharePoint

Regulatory affairs and clinical research professionals also need to make sure they stay updated on technological trends such as electronic submission standards, artificial intelligence (AI) applications, and data standards. Proficiency in programming languages allows these professionals to stay updated with these technological advancements, aiding in the implementation and management of advanced systems and AI-driven tools within their regulatory practices.
Developing Your Regulatory Affairs and Clinical Research Skills
If you're hoping to develop your skill set in regulatory affairs, it's important to know how to go about doing so. The best way to get started in a new field like regulatory affairs and clinical research is to gain as much hands-on experience as possible. Internships, co-ops, and educational opportunities can help you sharpen your knowledge and hone specific skills.
Here's a more in-depth overview of how to prioritize and develop your regulatory affairs skill set.
Prioritize the Skills You Should Put on Your Resumé
While ideally you'll obtain every skill required for your profession, these skills often take time to develop. For this reason, it's important to know which skills to prioritize and highlight on your resumé.
A good way to determine this is by considering the skills that others in a similar role list in their professional profiles. According to our analysis of job postings data, the top skills listed on regulatory affairs and clinical research professionals profiles include:
- Data Analysis
- Public speaking
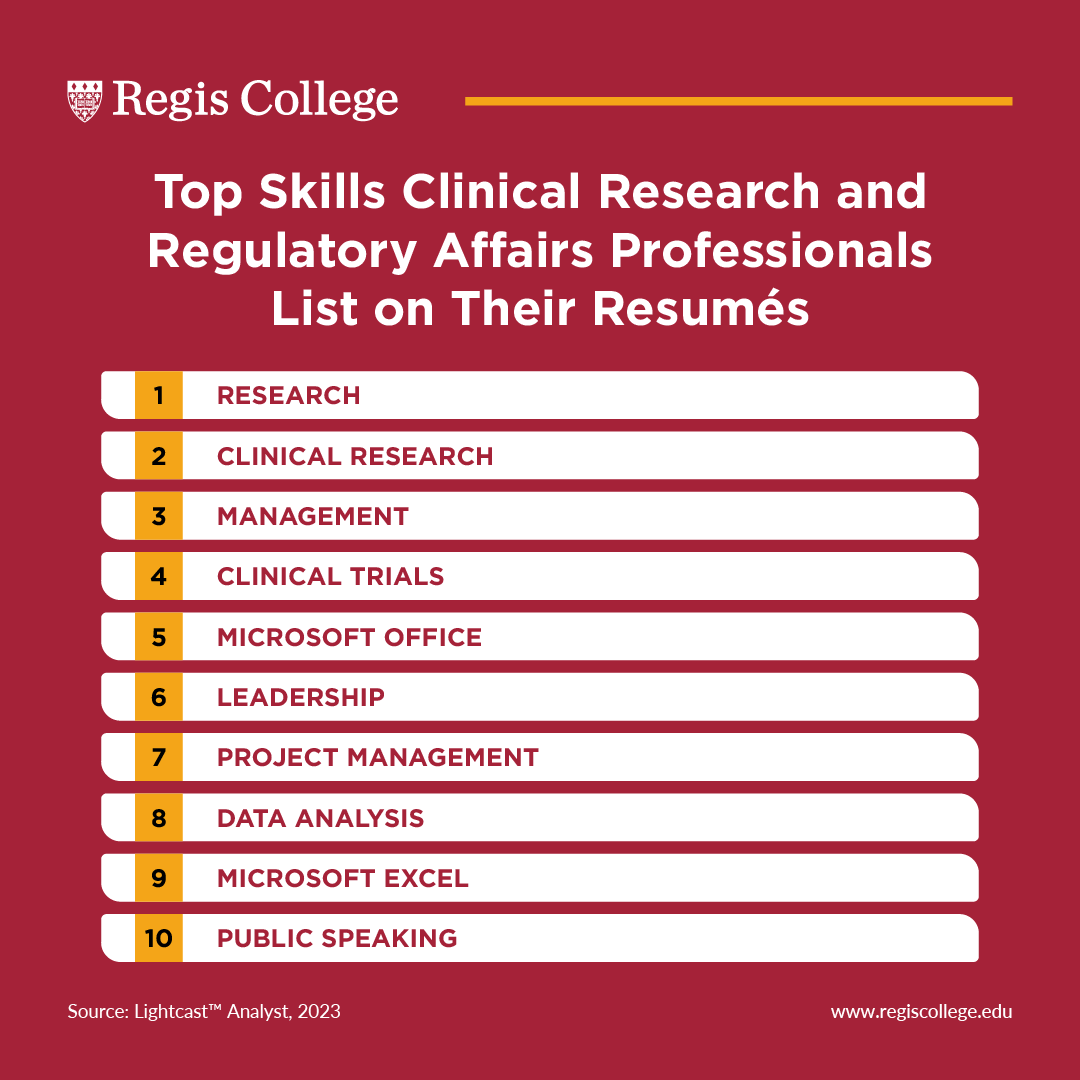
In order to stand out, it's important to ensure you have the same or similar competencies as others in your field. Otherwise, you might find yourself falling behind.
Advance Your Education
It's important not just to improve your skills, but to do so in a demonstrable way. One way to accomplish this is by advancing your education.
In both regulatory affairs and clinical research positions, advancing your education increases the scope of jobs you're able to apply for. Even if master's-level education can be substituted with experience, gaining additional education can help you stand out in your field and set you apart from other job candidates.
An excellent option for regulatory affairs professionals to advance their education is Regis College's Master of Science in Regulatory Affairs and Clinical Research Management (RCRM) .
This program will help you develop your skills in:
- Regulatory affairs: Regulatory affairs competencies such as pharmaceuticals and regulatory compliance.
- Quality compliance: For products to pass regulatory standards, they must comply with quality standards to evaluate the quality of new products or processes.
- Clinical research management: The management focus of this degree will help you obtain valuable interpersonal skills as well, helping you become a better leader overall.
In addition, through this program you'll have the opportunity to network with regulatory affairs and clinical research professionals in Boston , which is a hub for the healthcare and life sciences industries. You'll obtain real-world experience through valuable internship opportunities, and receive support from Regis in finding a job post-graduation in your desired field.
Ready to Gain the Tools Needed to Advance Your Career?
If you're ready to skill-up and advance your regulatory affairs or clinical research career, consider applying to Regis College's RCRM program. You'll have the opportunity to specialize in either clinical research management or regulatory affairs, allowing you to focus on the niche you want to pursue.
Furthermore, you'll be given the chance to work with faculty who are experts in this subject, and obtain familiarity with a wide range of applications of regulatory affairs and clinical research in the life sciences industry.

Related Blogs

Is a Master's in Regulatory Affairs and Clinical Research Worth It?
Wondering whether a master’s in regulatory affairs and clinical research is worth the investment? Here are several reasons the answer is yes.

What Is Regulatory Affairs?
Wondering what regulatory affairs is, and the role it plays in healthcare? Here’s an overview of regulatory affairs, and its importance in consumer safety.

Want to work in healthcare, but don’t have a clinical background? Here’s a list of seven behind the scenes medical jobs that don’t involve patient contact.
February 28, 2024
- 235 Wellesley Street, Weston MA 02493
- 781.768.7000
- © 2024
- Privacy Policy

What Does a Regulatory Specialist Do?

Industry Advice Regulatory Affairs
The regulatory affairs industry plays an important role in ensuring the safety of countless products that Americans use every day—from food and beverages to pharmaceuticals and medical devices to electronics and more. According to the FDA, 25 cents out of every dollar that Americans spend each year is spent purchasing a regulated product.
The ubiquity of regulatory work makes it an ideal field for anyone interested in using scientific methods and analysis to make a difference in the world around them.
Becoming a regulatory affairs specialist is one of the most common ways to break into the industry. Here, we explore the role and responsibilities of regulatory specialists, including working conditions and essential skills, as well as advice and tips for advancing in a regulatory career.
Download Our Free Guide to Advancing Your Regulatory Affairs Career
Learn how to navigate the discipline and accelerate your regulatory career.
DOWNLOAD NOW
What is a Regulatory Affairs Specialist?
A regulatory affairs specialist is someone who works to help a company or organization meet all state, local, federal, international, and industrial regulations that apply to their products. Most often, they work in the food and beverage , pharmaceutical, and medical devices industries , though many other companies—such as those that produce automotive or mechanical components, and even financial services—also require regulatory compliance.
Because most regulatory affairs specialists ( 76 percent , as of 2016) work directly in a regulated industry, most routinely interact with regulatory bodies and government agencies. Some of these professionals work directly for those regulatory agencies, including the Food and Drug Administration (FDA), Environmental Protection Agency (EPA), and the Federal Trade Commission (FTC), among others.
Key Responsibilities
A regulatory affairs specialist’s duties depend on a number of factors. The most important of these factors is the specific industry being worked in, as each industry is bound to its own set of regulations.
The responsibilities of these professionals also depend on the size of the organization. Regulatory specialists working for larger organizations may have a more narrowly-defined role compared to those who work at smaller organizations because larger organizations tend to have larger regulatory affairs offices or teams. A more focused role allows for increased specialization compared to organizations with smaller teams, which may require more flexibility from workers.
Some of the most common tasks and responsibilities of regulatory specialists include:
- Maintaining a deep understanding of new and existing regulations that may impact their organization’s products and processes
- Using that understanding to standardize all business operations and establish clear, documented protocols
- Explaining regulations, procedures, and policies to all employees and stakeholders as necessary
- Maintaining data and files for future reference, particularly in the event of an audit by a regulatory agency
- Reviewing marketing, legal, and technical documentation (including case files and clinical research reports) to assess compliance
- Recommending courses of remediation to help their company achieve the necessary levels of compliance
- Implementing internal audits of products and protocols to identify areas of weakness, and putting in place corrective measures
- Regularly reporting on compliance status and measures to both internal and external parties
- Preparing for and facilitating third-party audits as necessary
- Acting as a liaison between their organization and state, local, federal, and international agencies to submit required forms and paperwork
In addition to the responsibilities listed above, regulatory specialists are also often leveraged at various stages throughout the product development process to ensure compliance every step of the way, from research and development through manufacturing, marketing, and final approval.
Important Skills
If you’re interested in becoming a regulatory affairs specialist , developing the right skills can help. These include:
- Attention to detail: By nature, the work expected of a regulatory affairs specialist can be rather regimented, requiring that particular processes and protocols be followed at all times. That’s why attention to detail is so critical: One misstep can put lives and the organization’s reputation at risk.
- Analytical skills: While regulatory affairs specialists are not analysts, collecting and analyzing data to identify patterns and potential liabilities is still often a part of the job description for many.
- Communication skills: While regulatory expertise is essential to working in regulatory affairs, it’s useless if you cannot effectively communicate with those around you, whether they be coworkers, team members, vendors, or customers.
- Project management: Regulatory affairs is, in many regards, very similar to project management, requiring the planning and coordination of many moving pieces to achieve the desired result. (This is especially true during the development and launch of new products and services.) Therefore, skills like project and time management are essential.
- Domain expertise : To be effective in the role, a regulatory affairs specialist must be an expert in the domain of their company or organization. This means having a deep understanding not just of the products or services offered by the company, but also of the broader industry in which the company operates and the agencies that regulate it.
- Sense of mission: The best regulatory affairs specialists—those that truly make a difference—do not simply see regulatory affairs as a job. They understand the importance of their role in protecting consumers from danger and are compelled by this mission to reduce harm.
Career Advancement
If you’re interested in working in regulatory affairs , becoming a regulatory specialist is a great way to break into the field.
Generally speaking, these positions only require applicants to have earned a bachelor’s degree in a related field (product safety, biology, chemistry, and other sciences are popular fields). In exchange, specialists enjoy a competitive average salary of around $57,000, though this can reach as high as $85,000 depending on the industry and company.
While it is possible to gain an entry-level position with as little as a bachelor’s degree, according to the 2016 Compensation & Scope of Practice Report produced by the Regulatory Affairs Professionals Society (RAPS), 42 percent of employers prefer regulatory candidates who hold a graduate degree, and 45 percent of specialists hold a master’s degree.
Education levels become even more important as individuals move into more senior level positions, as these roles typically require a much deeper level of experience and understanding. According to the same RAPS report referenced above, graduate-level education is commonplace amongst senior professionals in the regulatory affairs industry. Forty-three percent of all vice presidents, 46 percent of all managers, and 40 percent of all directors hold at least a master’s degree.
It’s for this reason that many who wish to advance within the field will choose to pursue a graduate degree related to their industry—for example, a Master of Science in Regulatory Affairs .
Other important factors that can impact the trajectory of your career in regulatory affairs include:
- Experience: As in most fields, real, hands-on experience is critical for those hoping to advance in their careers. In the regulatory affairs industry, most VPs have an average of 19 years in the industry, directors have an average of 14 years, and managers have an average of 10 years. Taking every opportunity that you can to gain a wide variety of working experience—including internships and hands-on projects—can have major impacts on your career.
- Continuing education : Because the regulatory affairs industry is rapidly evolving as new regulations and technologies are created, it’s critical that regulatory professionals stay abreast of the latest developments affecting the industry.
- Network : Who you know in the industry can have a major impact on your career. Joining an organization like RAPS or The Organization for Professionals in Regulatory Affairs (TOPRA) can offer many networking opportunities, as can leveraging the professors and alumni from your degree program.
If you’re considering pursuing a career in regulatory affairs, including the skills and education required to get ahead, download our free guide below.

Subscribe below to receive future content from the Graduate Programs Blog.
About shayna joubert, related articles.

4 Ways to Stay Competitive in Regulatory Affairs

Emerging Trends in Regulatory Affairs in 2022

How to Stay Updated on Regulatory Changes
Stay in-demand.
64% of regulatory affairs professionals hold an advanced degree. (RAPS, 2020)
Regulatory Affairs Graduate Programs
Become qualified to manage global regulatory processes and develop cutting-edge products in healthcare and food safety.
Most Popular:
Tips for taking online classes: 8 strategies for success, public health careers: what can you do with an mph, 7 international business careers that are in high demand, edd vs. phd in education: what’s the difference, 7 must-have skills for data analysts, in-demand biotechnology careers shaping our future, the benefits of online learning: 8 advantages of online degrees, how to write a statement of purpose for graduate school, the best of our graduate blog—right to your inbox.
Stay up to date on our latest posts and university events. Plus receive relevant career tips and grad school advice.
By providing us with your email, you agree to the terms of our Privacy Policy and Terms of Service.
Keep Reading:

Top Higher Education Conferences To Attend in 2024

Grad School or Work? How To Balance Both

Is a Master’s in Computer Science Worth the Investment?

Should I Go to Grad School: 4 Questions To Consider
- RECRUITMENT

Clinical Research, Pharmacovigilance and Regulatory Affairs
Program details.
- Type: Post-Graduate Diploma
- Start Date: September 30, 2024, more
- Duration: 45 Weeks
- Campuses: Toronto and Mississauga
- Admission Requirement:
- » BSc. (or equivalent) or higher level of education.
Course Outline
- CR09 The Canadian Pharmaceutical Industry: Big Picture
- PRA101 Introduction to Regulatory Affairs
- GMP1001 Introduction to Good Manufacturing Practices – Level I
- CR013 Clinical Pharmacology and Clinical Safety Assessments
- CR014 Good Clinical Practices (GCP), Research Ethics and Clinical Research Regulations

- PRA302 Regulatory Affairs Generic Drugs
- PRA204 Regulatory Affairs Medical Device
- CR019 Organization of Clinical Trials and Clinical Monitoring Plan Development
- CR020 Clinical Project Management and Project Chart Development
- CR021 Clinical Data Acquisition and Data Management
- CR023 Drug Lifecycle Safety Management and Pharmacovigilance Compliance
- TWC3001 Technical Writing and Scientific Communication
- CR025 Clinical Study Protocol and ICF Development
- PRA305 Global Regulatory Affairs
- PRA205 Regulatory Affairs for Natural Health Products
- CR028 Global Clinical Research and Pharmacovigilance
- PRA202 Regulatory Affairs for Clinical Research and Preclinical - Drugs
- CR030 Clinical Research, Drug Safety and Pharmacovigilance Project
- PRA203 Regulatory Affairs Biotech/Biologics
Program Overview
The demand has never been greater for professionals who can help companies ensure compliance with applicable laws and regulations in the development and commercialization of new drugs and healthcare products. The Clinical Research, Pharmacovigilance and Regulatory Affairs program will provide you with knowledge and insight into the most recent developments in the clinical research, pharmacovigilance and regulatory affairs field. You will learn current regulatory and pharmacovigilance research current clinical research topics including Good Clinical Practices (GCP), regulatory submission, pharmacovigilance regulations and Good Pharmacovigilance Practices (GVP), and quality assurance concepts in clinical trials and pharmacovigilance. As a student in this program, you will benefit from an applied and practical approach to training and develop a network of industry contacts ahead of graduation.
CRPRA Program Brochure
Career opportunities.
Graduates of the C linical Research, Pharmacovigilance and Regulatory Affairs Post-Graduate Diploma Program can pursue careers in the Pharmaceutical, Biotechnological, Medical devices, Cosmetics, Natural health Products, and allied industries. Trained and qualified RA professionals are in demand for pharmaceutical, biotech, medical device and natural health product companies as they are needed to navigate the intricacies of regulatory submissions for new products.
AAPS graduates have been hired as:
- Clinical Research Coordinator
- Pharmacovigilance Data Management Associate
- Clinical Research Associate
- Pharmacovigilance Associate
- Clinical Data Management Associate
- Clinical Research Project Leader
- Pharmacovigilance Project Leader
- Clinical Research Monitor
- Medical Information Associate
- Pharmacovigilance Officer
- Quality Assurance in Clinical Research
- Quality Assurance in Pharmacovigilance
- Auditor in Clinical Research and Pharmacovigilance
- Quality Assurance Associate/Technician
- Auditor/Inspector
- Technical Writer
- Document Reviewer
- Regulatory Affairs Associate
- Regulatory Compliance
- Regulatory Operations Associate
AAPS graduates of Clinical Research, Pharmacovigilance and Regulatory Affairs post-graduate diploma qualify for Certified Clinical Research Professional – CCRP Certification awarded by Society of Clinical Research Associates (SOCRA). For more detail contact AAPS at 416-502-2277
For more information on benefit of CCRP certification please visit https://www.socra.org/certification
Organizations who have hired AAPS Graduates

More Companies

Certifications
Graduates will receive a Post-Graduate Diploma on Clinical Research, Pharmacovigilance and Regulatory Affairs. This program is approved as a vocational program under the Ontario Career Colleges Act, 2005 . The Ontario Career Colleges Branch is part of the Ministry of Colleges and Universities and the Superintendent of Ontario Career Colleges is an appointed position by the Minister of Colleges and Universities.
AAPS Graduates will also qualify for Certified Clinical Research Professional - CCRP Certification awarded by Society of Clinical Research Associates (SOCRA).
To register for in-class, contact us via telephone at 416-502-2277 or by email at [email protected].
In Class Registration Process
Get the Reddit app
Clinical research is a branch of healthcare science that determines the safety and effectiveness (efficacy) of medications, devices, diagnostic products, and treatment regimens intended for human use. These may be used for prevention, treatment, diagnosis or for relieving symptoms of a disease.
Regulatory Coordinator vs Regulatory Affairs Associate/PM
Hi. Newbie here, seeking career advice.
My Background: I have 15+ clinical testing QA experience, 5+ project management experience. Have a graduate degree in Quality Assurance & Regulatory affairs, and have my PMP. I've been unsuccessfully trying to break into clinical research for the last 6+ months.
My question: To get into clinical trial management , am I better off focusing on a regulatory coordinator position, or regulatory affairs associate/project manager position?
It seems to me that both roles are in clinical research but differ where the coordinator is at the site level, and the affairs associate/PM roles are at the CRO/sponsor - and is considered a more senior, higher paying position.
But just because the reg affairs roles are more senior and pay better, will I be able to move from there to a CRA or CTM role? Or will I be stuck, and require an entry-level role in the future? If I need to take an entry level position, I feel like now is the time to do it.
Thanks for all the help!!
By continuing, you agree to our User Agreement and acknowledge that you understand the Privacy Policy .
Enter the 6-digit code from your authenticator app
You’ve set up two-factor authentication for this account.
Enter a 6-digit backup code
Create your username and password.
Reddit is anonymous, so your username is what you’ll go by here. Choose wisely—because once you get a name, you can’t change it.
Reset your password
Enter your email address or username and we’ll send you a link to reset your password
Check your inbox
An email with a link to reset your password was sent to the email address associated with your account
Choose a Reddit account to continue

IMAGES
COMMENTS
Differences between clinical research associate and regulatory affairs specialist duties and responsibilities Clinical Research Associate Example Responsibilities. Manage, schedule and train up to 15 CRAs. Recruit patients, attain patient inform consent form, educate subjects on compliance, and ensure patient safety per ICH guidelines.
At least with an RA spot, you can start to learn the submission process, query process, regulatory strategy, interactions with reg bodies, learn the clinical development process as a whole, and get to sit on study/program team meetings. You should easily be able to work your way to a manager after a solid 1-2 years where the real money starts.
Here are the pros/cons: Pros: very good job security, satisfying work, high demand, and good pay. Reg involves work that puts you in touch with many departments (CMC, R&D, commercial, drug safety, etc) Cons: for the highest level jobs you'll need a doctoral degree plus experience. Hard to transfer from Reg affairs to clinical research.
Regulatory affairs and clinical development are parts of the biotech pipeline that come after discovery research and before the product can be used out in the world (which is most of that pipeline!) In this way, scientists in regulatory and clinical careers are important gateways. The main purpose of this gateway is to keep people safe ...
Regulatory affairs play a pivotal role in ensuring the safety, efficacy, and quality of pharmaceutical products and medical devices. In the field of clinical research, regulatory affairs professionals are at the forefront of ensuring compliance with the laws and regulations set forth by regulatory agencies such as the FDA (Food and Drug Administration), EMA (European Medicines Agency), MHRA ...
Without an MD or other clinical degree your career progression in clinical ops is severely limited. Regulatory Affairs values experience over any formal education. The RA education programs are not valued in the industry. Get a PhD in ANY scientific field or a PharmD and you'll easily get a job in RA, though many only have masters degrees.
Conclusion. The role of regulatory affairs in clinical research is indispensable. Regulatory affairs professionals ensure that clinical trials are conducted ethically, safely, and in compliance with all relevant regulations. Their work is critical to the development of new medical treatments and the advancement of medical science.
Answer: Clinical research administration and regulatory affairs are two related jobs that are integral to the rigorous process of bringing pharmaceuticals and medical devices from the discovery phase to market. Clinical research administration involves direct oversight of clinical trials that test the safety and efficacy of new medications ...
A regulatory affairs and clinical research education is a gateway to diverse career paths beyond the traditional roles, especially if you're drawn to leadership positions. While many graduates opt for careers directly related to regulatory affairs, such as regulatory affairs specialists or clinical research coordinators, the versatile ...
Associate VP, Clinical Development, Ferring Pharmaceuticals (UCSF Alumnus, Biomedical Sciences 2007) Brandi Saunders, MS Principal Consultant, Opus Regulatory (UCSF Alumna, Nursing 2004) Regulatory and Clinical Affairs questions answered in November 2020 by: Leslie Ann Cruz, PhD ... Research the people you will be interviewing with. As with #4 ...
Regulatory affairs specialists must understand the roles and responsibilities of clinical researchers and vice versa. This continuous flow of information and understanding are what brought Jennifer Cuvin to our Professional Program in Regulatory Affairs. "Because clinical operations work alongside regulatory associates," Jennifer says, "I ...
Master's Degree vs. Certificate in Regulatory Affairs. As you start a career in regulatory affairs or clinical research, you'll likely come across two distinct educational paths: obtaining a master's degree or earning a graduate certificate.Both options provide graduate-level education that can expand your career opportunities.
The online MSHS in Regulatory Affairs and Clinical Research Leadership program is ideal for regulatory affairs, clinical research and health care professionals looking to lead regulatory strategy and therapeutic product development in the evolving field of health care. ... Clinical Research Associate, $73,307 avg. salary 7; Clinical Data ...
Auditing. Regulatory compliance. Drug development. Pre-clinical development. Biology. Obtaining the necessary hard skills for a career in clinical research or regulatory affairs isn't always easy. It typically requires prior working experience or advanced education in a clinical research or regulatory affairs program.
There is a wide variety of careers in the regulatory affairs field. Regulatory professionals carry titles such as: Regulatory affairs specialist. Regulatory affairs manager. Regulatory affairs director. Compliance specialist. Food safety inspector. Clinical research associate. Director of quality assurance.
A regulatory affairs specialist is someone who works to help a company or organization meet all state, local, federal, international, and industrial regulations that apply to their products. Most often, they work in the food and beverage, pharmaceutical, and medical devices industries, though many other companies—such as those that produce ...
Thanks in advance :) in the US: I'd say with a PhD, you'd be more marketable and also more able to switch between reg affairs and clinical research. You may still be able to get positions in both with a masters, but you'd be more limited IMO in the clinical research side without a PhD. I don't think you can really go wrong with reg affairs.
AAPS graduates have been hired as: AAPS graduates of Clinical Research, Pharmacovigilance and Regulatory Affairs post-graduate diploma qualify for Certified Clinical Research Professional - CCRP Certification awarded by Society of Clinical Research Associates (SOCRA). For more detail contact AAPS at 416-502-2277.
Shannon Strom is a senior regulatory scientist and associate director of regulatory operations at CATO Research in Durham, North Carolina. She did her Ph.D. in pharmacology at the University of North Carolina (UNC), Chapel Hill. "One of my favorite experiences in my lab had been a collaboration with an M.D./Ph.D. who had a direct connection to ...
The ICH E6(R2) guideline does, however, include a definition of a regulatory authority and it is useful to reflect on it because it sets the stage for how these entities fit, from a good clinical practice standpoint into the clinical trial arena. The roles of regulatory authorities in the oversight of clinical trials have been an evolutionary ...
Currently a Sr. CRA, however, I've been looking for an exit strategy into clinical data manager or regulatory affairs positions. After 5 months of searching and applying I've come up short. However, I've recently been offered a position as a regulatory affairs associate.
There are two types of organizations that use the acronym CRO : contract research organizations and clinical research organizations. Since new drug innovators may use one or both types of CRO s, both are included in this document. ... regulatory affairs, and quality assurance. Contract research organizations play a pivotal role in drug ...
To earn this certification, you must have one of the following: At least two years of clinical research experience or 3,500 hours of part-time experience in the past five years. A degree in clinical research and at least one year of full-time experience. A certificate in clinical research, a bachelor's or associate degree in health science ...
At a site a clinical regulatory person does IRB submissions and maintains the site binders. They can become CRC or even trial manager at the site. It's good to get your foot in the door or if you just want a non-patient facing role at a site. Regulatory Affairs has a very defined career path but works on the sponsor side typically.
Clinical research is a branch of healthcare science that determines the safety and effectiveness (efficacy) of medications, devices, diagnostic products, and treatment regimens intended for human use. ... Regulatory Coordinator vs Regulatory Affairs Associate/PM . Career Advice Hi. Newbie here, seeking career advice. My Background: I have 15 ...