CASE REPORT article
Case report: successful treatment of human diabetic foot ulcer using low-intensity diagnostic ultrasound combined with microbubbles: two cases.

- 1 Department of Ultrasound, the General Hospital of Western Theater Command, Chengdu, China
- 2 Department of Endocrinology, the General Hospital of Western Theater Command, Chengdu, China
Background: Diabetic foot ulcer (DFU) is one of the serious complications of diabetes, which has high disability rate and mortality. Low-intensity ultrasound combined with microbubbles in blood circulation can enhance the blood perfusion effect of local soft tissue, which has the potential to promote the healing of diabetic ulcer. Here, we report how this method was used to help the healing of two patients with chronic refractory DFUs.
Case Presentation: In case 1, a 56-year-old man with 3-years history of type 2 diabetes had a 3.0×2.0 cm ulcer which infected with staphylococcus aureus on his right calf for more than half a month. In case 2, a 70-year-old man with 10-years history of type 2 diabetes presented with an 8-month right heel ulcer that developed to 7.5×4.6 cm. And he also had hyperlipidemia, hypertension, and renal impairment. Both patients were enrolled in our study to receive treatment of low-intensity diagnostic ultrasound (LIDUS) combined with microbubbles. They were discharged after a 20-minute daily standard treatment for 7 consecutive days. The ulcers in both cases completely healed in 60 days and 150 days, respectively, and haven’t recurred for more than one year of follow-up.
Conclusion: It is feasible, safe, and effective to use commercial LIDUS combined with commercial microbubbles in the treatment of diabetic lower extremity ulcers. This study may provide an innovative and non-invasive method for the treatment of DFUs.

Introduction
Diabetic foot ulcer, as one of the serious complications of diabetes, has brought heavy economic and public health burden to the society due to its high incidence (15-25%) ( 1 ), high disability rate and high mortality ( 2 ) in diabetic patients.
Microcirculatory dysfunction is an important cause of DFU. On one side, hyperglycemia and hyperinsulinemia promote characteristic extensive endothelial hyperplasia, basement membrane thickening, and even calcification in arterioles, leading to ischemia-hypoxia and poor perfusion in foot soft tissue ( 3 ). On the other, hyperglycemia and oxidative stress lead to endothelial dysfunction, characterized by impaired auto-regulation of micro vessels and a blunted response to vasodilatory stimuli, thereby exacerbating functional perfusion defects in the limbs ( 4 ).
Endovascular shear force is an important means to regulate endothelium-derived vasoactive substances and control micro vasodilation ( 5 ). According to this, a series of related drugs and modified endogenous active substances have been developed to treat tissue ischemia through improving microcirculation perfusion.
Low-intensity pulse ultrasound, is a kind of ultrasonic energy mainly with mechanical effect, but not thermal effect. The shear force, micro jet and shock wave generated by ultrasonic pulse produce a series of physical and biological effects, which are widely used in the therapeutic field. Perfusion effect is one of these effects, which is to enhance local blood perfusion in tissues by setting appropriate acoustic parameters ( 6 ). The microbubbles in the circulation, as cavitation nuclei, could make the ultrasound produce very high shear force and multiply the effect of local blood flow enhancement. Therefore, when low-intensity ultrasound is combined with microbubbles, it has a very good potential for the treatment of tissue ischemic diseases.
In this report, we presented two complete healing cases of refractory DFU treated by commercial LIDUS combined with commercial microbubbles for the first time.
Materials and methods
A GE LOGIQ 9 ultrasound scanner (GE Healthcare, Waukesha, WI) equipped with a 9L Linear array probe (GE Healthcare) was used for both conventional ultrasonography and Contrast-Enhanced Ultrasonography (CEUS). In conventional ultrasonography, thyroid imaging mode was used with a frequency of 9MHz and an imaging depth of 4cm. In CEUS, “Contrast” key was clicked.
An Acuson S2000 ultrasound scanner (SIMENS Healthcare, Erlangen, Germany) equipped with a 9L4 Linear array probe was used for all treatments. Contrast pulse sequencing (CPS) mode were used to monitor microbubble perfusion and an intermittent flash of high MI impulses. The frequency of flash was set at 4 MHz, Imaging depth at 4 cm, with a frame rate of 50 frames per second and an MI of 0.86 (79% acoustic output power).
Same CEUS imaging sections of the ulcerative and surrounding soft tissue before and after treatment were used for perfusion evaluation, chartered with adjacent vessels or bony structures. All parameters of ultrasound were consistent in both patients during diagnosis and treatment.
The microbubbles used for ultrasonic diagnosis and treatment were SonoVue (Bracco Imaging Scandinavia AB, Oslo, Norway), a commercial ultrasound contrast agent. The microbubble suspension with a concentration of 11.8 mg/mL were prepared according to the manufacturer’s instructions, with 59 mg sulfur hexafluoride lyophilized powder mixed with 5 mL of normal saline. A 140 ×110 ×7 mm acoustic coupling pad (Foshan SiEn Technology Co., LTD., Guangzhou, China) was used during imaging and treatment procedures for better coupling.
After routine clinical debridement of the wound, the acoustic coupling pad was placed on the ulcerated skin area. CEUS was first performed on local tissue, and 2.4 ml microbubble suspension was injected rapidly through the cubital vein, followed by flushing with 5 ml normal saline. CPS angiography combined with microbubble Flash mode was used for treatment: “Microbubble Flash → Microbubble Contrast → Microbubble Flash → Microbubble Contrast”. In the first 5 minutes, the remaining circulating microbubbles from previous CEUS were used to mediate ultrasound therapy. Then another 5 ml of the prepared microbubble suspension was taken and injected slowly and continuously through the vein for 10 min. Finally, the remaining circulating microbubbles were used again to mediate ultrasound treatment for 5 min, and the total time of ultrasound treatment was 20 min. The ultrasound treatment cycle was 7 days, once a day, and the treatment process was the same for each time. Follow-up observation was conducted for 6 months.
Case presentation
A 56-year-old man with 3-years history of type 2 diabetes fell to the ground while cycling two weeks ago, resulting in a skin ulceration on his right calf. He received basic debridement and daily dressing change at local hospital but the ulcer did not heal. Subsequently, the patient was admitted to the department of Endocrinology in our hospital. The patient was not taking any medication at admission.
Physical examination showed an ulcerated surface on the patient’s medial right calf, about 3.0 ×2.0 cm in size, with a depth of about 6 mm ( Figure 1A ). The granulation tissue was relatively fresh, with a little dark red bloody exudate on the surface, and the surrounding soft tissues were red, swollen and slightly tender. Laboratory examination showed increased fasting blood glucose (16.2 mmol/L), increased HbA1c (7.10%), normal liver and renal function indexes. Secretion culture from the ulcer indicated an infection of Staphylococcus epidermidis ( Table 1 ). Magnetic resonance imaging (MRI) examination showed that the ulcer did not involve bone tissue ( Figure 1B ). Color Doppler Flow Imaging (CDFI) showed no significant abnormalities in peripheral arteries ( Figure 1C ). Ankle-brachial index (ABI) was normal (ABI=1.20), Current perception threshold (CPT) was 0.00, and there was no abnormal sensation ( Table 1 ). Based on these evidence, the patient preliminary diagnosed as diabetic foot ulcer (Grade 3 of Wagner classification).
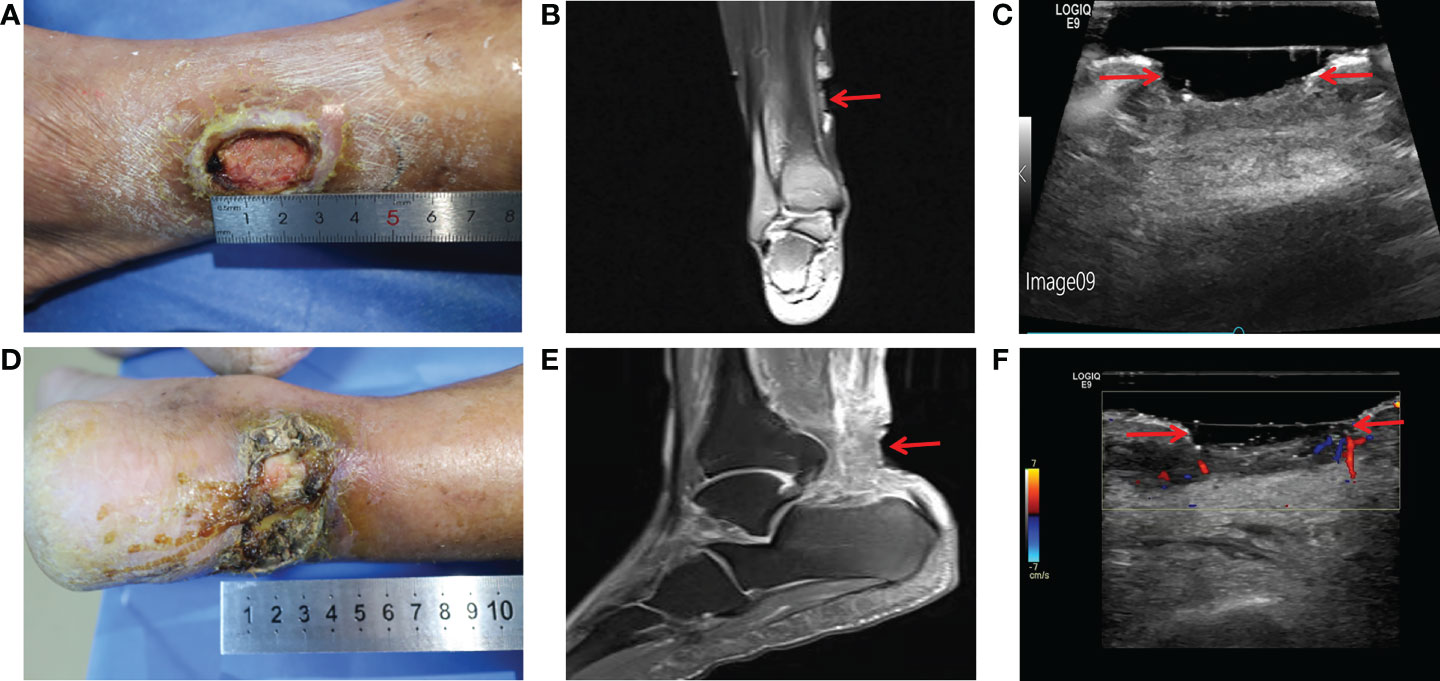
Figure 1 Imaging diagnosis of diabetic lower extremity ulcers before treatment for case 1 (A-C) and case 2 (D-F) . (A) Picture showed an ulcerated surface on the inner skin of the right calf, about 3.0×2.0 cm in size and 6 mm in depth; (B) MRI showed a local subcutaneous soft tissue defect at the medial margin of the right calf, with swelling in the margin and adjacent soft tissue space; (C) Gray-scale sonography showed a heterogeneous low echo area in the subcutaneous soft tissue of the medial side of the right calf; (D) Picture showed an ulcerated surface in the skin of the right heel, about 7.5 × 4.6 cm in size and 4 mm in depth, with necrosis and exudation; (E) MRI showed extensive swelling of the soft tissue and fascia in the lower part of the right calf with unclear and disordered layers; (F) CDFI showed local skin defects and discontinuity in the skin of the right heel. The blood flow signal in the low echo surface was not obvious, and a little blood flow signal could be seen in the periphery. The red arrows indicate the ulcer defects.
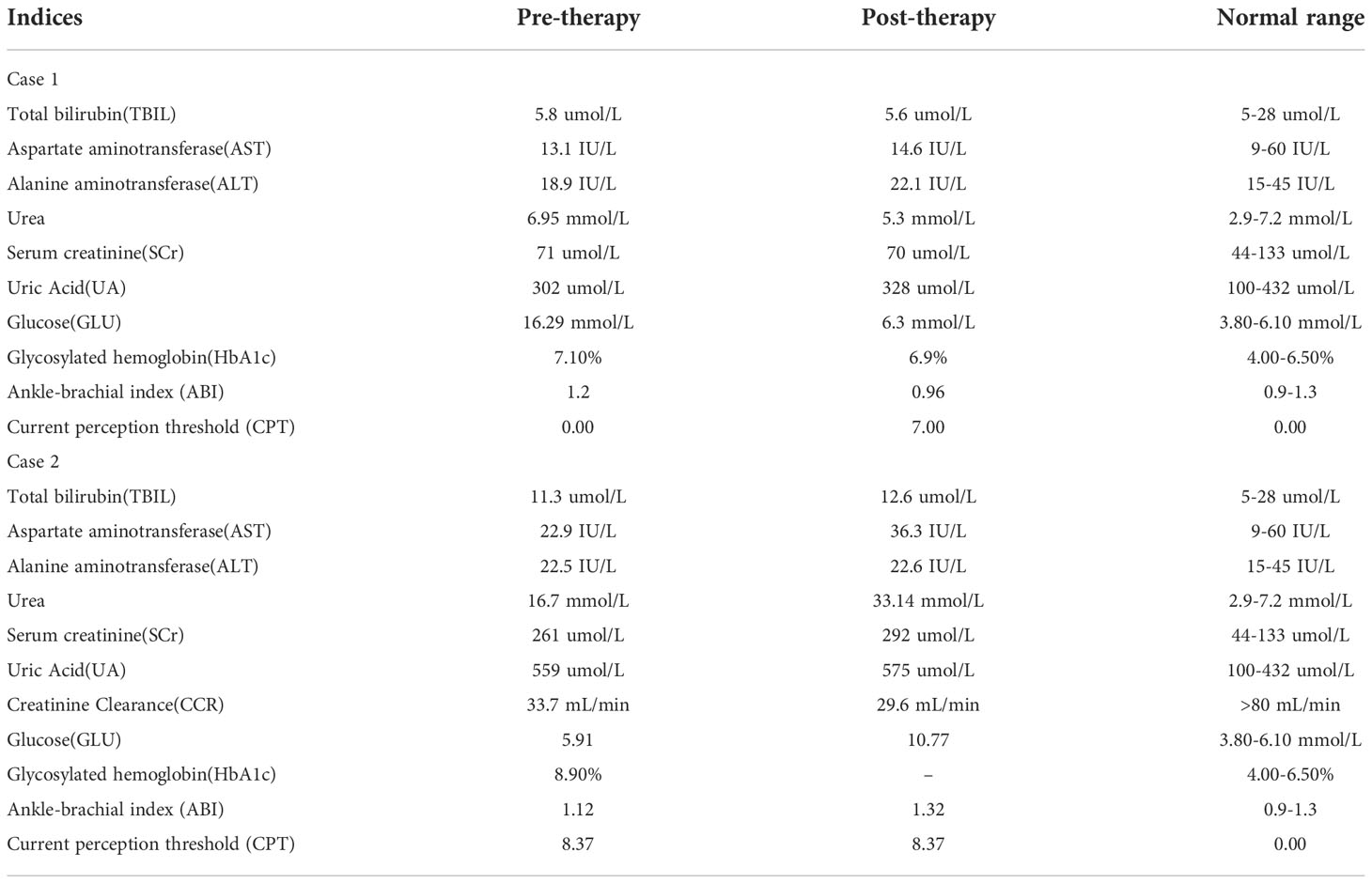
Table 1 Details of blood routine, blood glucose level and other testing.
After admission, the patient received antibiotic therapy for 8 days (intravenous cefotiam hydrochloride, 1g/8h, once a day). The ulcer wound dressing were changed once a day. For blood glucose control, the patient also received subcutaneous injection of recombinant human insulin (4 IU, three times a day), before each meal, subcutaneous injection of protamine human insulin (10 IU, once a day), at 22:00 every day, oral metformin hydrochloride sustained-release tablet (0.5 g, twice a day), oral sitagliptin phosphate tablet (100 mg, once a day). LIDUS combined with microbubbles therapy was performed once a day from the 5th day of admission ( Figure 2A ). On the 8th day of anti-infection, there was no purulent secretion in the wound, and the redness and swelling of the surrounding block were alleviated. On the 12th day of blood glucose control, the blood glucose level reduced to 6.3 mmol/L ( Table 1 ). On the 12th day after enrolling in the LIDUS therapy, the ulcer area also decreased from 3.0 ×2.0 cm to 2.8 × 1.4 cm and its depth decreased from 6 mm to 4 mm, filled with granulation tissue, no purulent exudation was present. The patient was then discharged and continued to receive standardized blood sugar control treatment. Follow-up found the ulcer skin recovered 60 days after enrolling in LIDUS therapy ( Figures 2B–F ). Liver and kidney function were reexamined after ultrasound combined with microbubble treatment, and no abnormalities were found. Ankle brachial index was 0.96, slightly decreased. The CPT grade was 7.0, indicating mild hypoesthesia ( Table 1 ). After discharge on the 12th day, the following medications were given to control glycemic for the next 15 days, subcutaneous injections of recombinant insulin lyprol (10 IU-8 IU, twice a day), before breakfast and dinner, oral metformin hydrochloride sustained-release tablet (0.5 g, twice a day), oral sitagliptin phosphate tablet(100 mg, once a day). Subsequently, the patient stopped glucose-controlling drugs by himself, and wound dressing was changed daily. To be clear, the timeline of entire treatment process of this case was presented in Figure 3 .
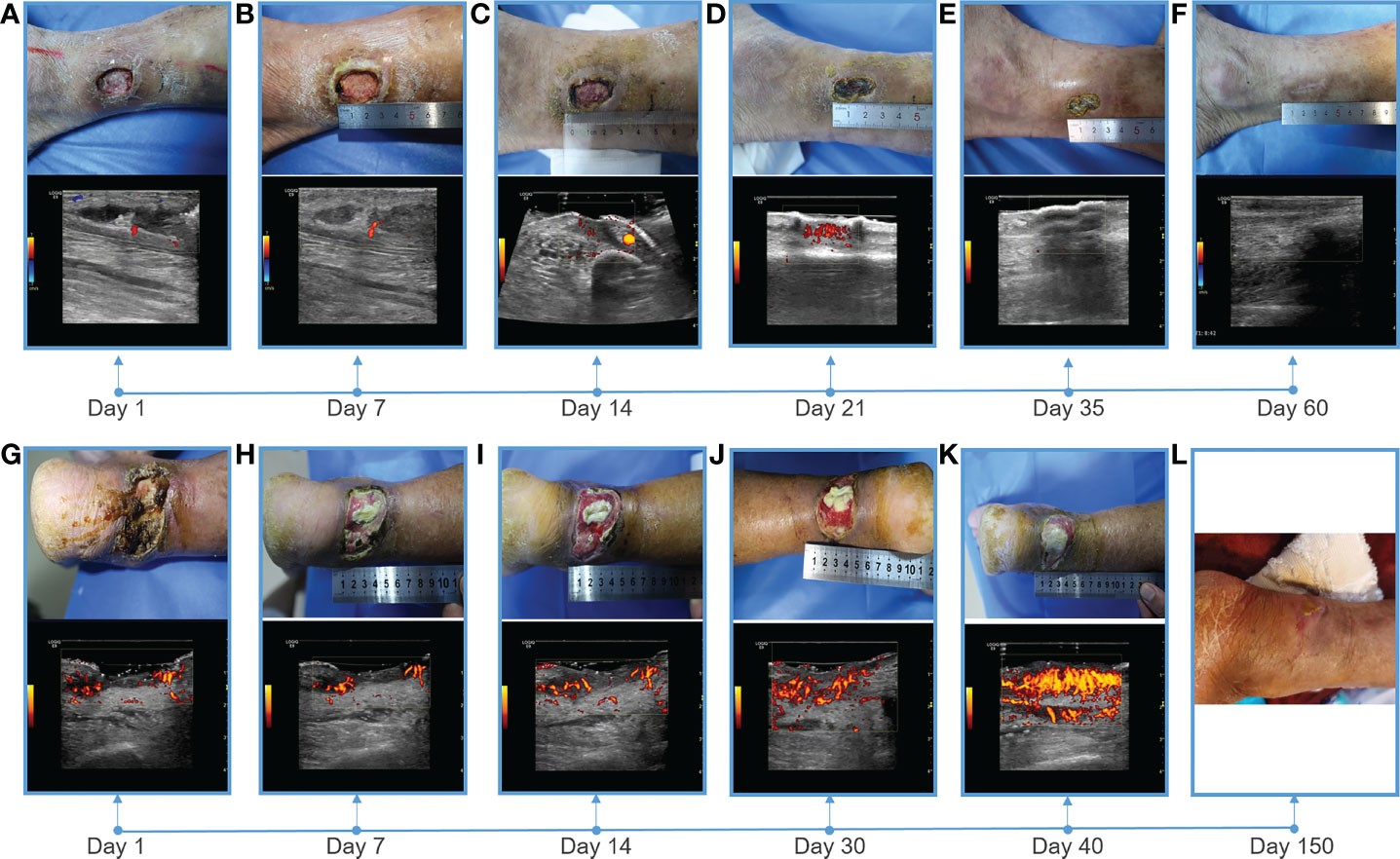
Figure 2 Showing of skin wounds and CDFI or PDI ((Power Doppler Imaging)) ultrasound imaging for progress in the treatment of diabetic lower extremity ulcers in two cases. Wound conditions at the (A) 1th, (B) 7th, (C) 14th, (D) 21th, (E) 35th, and (F) 60th day of post therapy showed the gradual healing of ulceration for case 1; Wound conditions at the (G) 1th, (H) 7th, (I) 14th, (J) 30th, (K) 40th, and (L) 150th day of post therapy showed the gradual healing of ulceration for case 2. PDI showed that the blood flow of the soft tissue around the ulcer gradually increased, and the blood flow was very abundant before healing.
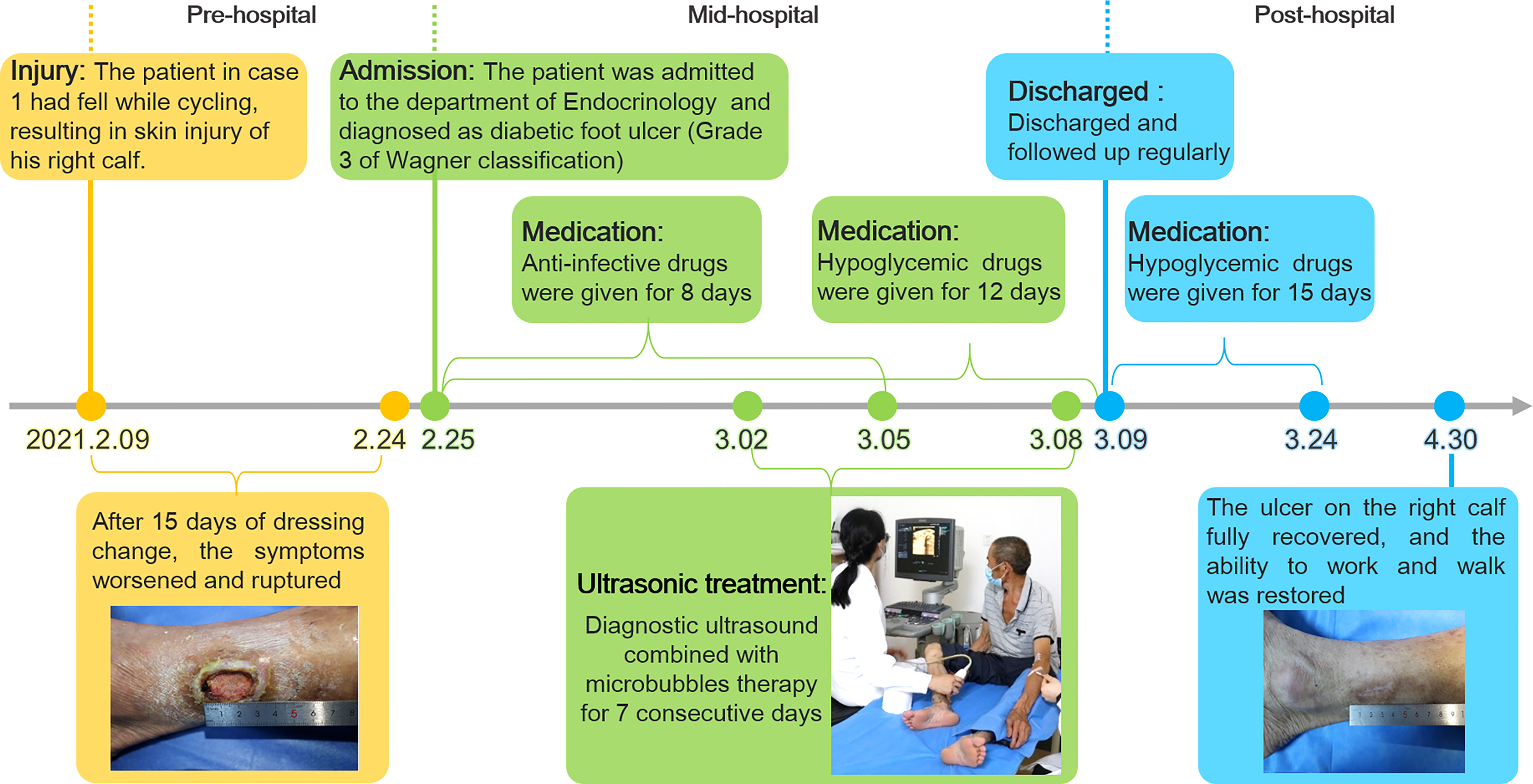
Figure 3 The timeline of the treatment process of case 1 from the day of injury to the day of healing.
A 70-year-old male patient diagnosed with type 2 diabetes for more than 10 years had poor glycemic control due to irregular medication. The patient found an ulcer on his right heel without any known injuries eight months ago. After removing the black scab on the surface of the ulcer by himself, the ulcer was getting worse and the patient was subsequently admitted to the Endocrinology Department of our hospital. Since the onset of the ulcer, the patient had complained about a progressively deterioration in mental, physical, appetite and sleep. The patient also had a history of alcohol abuse for more than 30 years (about 500mL strong wine a day).
Physical examination revealed a skin defect about 7.5 × 4.6 cm in size and 4 mm in depth, with black crusts and yellowish exudate, surrounding skin redness and swelling, and pain when walking and pressing the wound ( Figure 1D ). Laboratory examination showed normal fasting blood glucose (PP 5.91 mmol/L), increased HbA1c (8.90%), normal liver function, and Renal insufficiency (blood urea (16.76 mmol/L, serum creatinine 261 umol/L, and serum uric acid 559 umol/L) ( Table 1 ).Blood pressure test showed hypertension (160/110 mmHg). MRI examination showed that the ulcer did not involve bone tissue ( Figure 1E ). CDFI showed mild atherosclerosis of lower extremity arteries ( Figure 1F ). Ankle-brachial index was in the normal range, and the CPT grade was 8.37, suggesting moderate hypoesthesia. Based on these evidence, the patient preliminary diagnosed as type 2 diabetes, diabetic foot, hyperlipidemia, hypertension and renal impairment in the outpatient department of endocrinology of our hospital.
Heel ulcer debridement was performed on the patient first ( Figure 2G ). After debridement, the ulcer surface showed no obvious granulation tissue and light red color, and the Achilles tendon was partially necrotic and pale color with partial yellowness. After 7 consecutive days of ultrasound combined with microbubble therapy, the ulcer area did not change significantly ( Figure 2H ). On the 7th day, the granulation tissue grew obviously, and the tendon tissue grew slightly ( Figure 2H ). On the 14th day, the wound contracted slightly and granulation tissue grew with bright red color ( Figure 2I ). On the 30th day, the granulation tissue tended to fill the wound, and a little epidermal tissue grew around it ( Figure 2J ). On the 40th day, the wound contracted and became slightly smaller, granulation tissue protruded from the skin surface, and the tendon tissue was completely repaired with normal color ( Figure 2K ). During the LIDUS therapy, the patient’s local pain and itching gradually increased. Subsequently, the patient was to be treated by skin grafting in the burn department. However, due to the long-term high blood glucose (about 10 mmol/L), the surgeon suggested controlling blood glucose before operating. With no other treatment, after 150 days, the wound was covered with epidermis and the ulcer was basically healed ( Figure 2L ). There was no significant change in liver and kidney function before and after ultrasound treatment, ankle-brachial index increased (ABI=1.32), indicating arterial stiffness, and the CPT grade was 8.37, indicating moderate hypoesthesia ( Table 1 ). The patient was treated only in the outpatient department, and glycemic control was simply by oral metformin hydrochloride sustained-release tablets (0.5g, twice a day), which was not effective.
The therapeutic effect in 2 patients was positive and encouraging. The size and depth of the ulcer determined the time to cure, and the ulcer completely healed in 60 to 150 days, and the patients were able to live independently. The safety of the treatment process was also verified. There was no significant difference in liver and kidney function in two patients before and after LIDUS therapy. In addition, there was no ecchymosis on the local body surface, and no thrombosis and other adverse events occurred in local veins and arteries. What is noteworthy is patient 2, whose wound was large and blood sugar fluctuated for a long time and remained high. After careful surgical evaluation, skin grafting was finally abandoned to close the wound. Unexpectedly, after 150 days of slow growth, the skin healed on its own. Both patients were followed up for more than one year and had no recurrence of ulcers. At a recent follow-up visit, Patient 1 said that compared with other methods he had known, receiving our treatment was like undergoing ultrasound examination, which was painless, non-invasive, easy to adhere to, and had definite efficacy, and he was happy to share this treatment with other patients.
To our knowledge, this was the first human trail that used LIDUS combined with microbubbles to treat diabetic foot ulcer. Almost all previous studies on ultrasonic treatment of diabetic foot ulcer were in vitro or preclinical ( 7 – 9 ). Until now, the study of low-intensity ultrasonic cavitation in the treatment of human diabetic foot ulcer has not been reported. The only known clinical application of LIDUS plus microbubbles is in the field of tumor therapy. Kotopoulis ( 10 ) and Liuzheng ( 11 ) have respectively used this method to enhance microcirculation blood supply to pancreatic and breast cancer tumors, and improve the efficacy of chemotherapy.
Blood flow enhancement by LIDUS is the premise of this clinical experiment, which was found in our previous animal studies. When MI was set to 0.3, 5 minutes after ultrasound combined with microbubbles irradiation for VX2 tumor, the tumor blood supply was significantly increased by contrast enhanced ultrasound by a direct visualization method and TIC curve quantitative analysis ( 12 ). Similarly, in this study, after the soft tissue around DFU was treated for 20 minutes, its blood perfusion was observed increased by direct visualization, and reached a higher peak intensity (-46 to -42dB) by quantitative analysis in a shorter time and decreased more slowly after treatment. The results showed that vascular resistance of muscle tissue decreased and blood perfusion increased after treatment. In vivo studies have reported that low-intensity ultrasound combined with microbubbles irradiating muscle tissue for more than 10 minutes can reverse ischemia up to 24 hours ( 6 ).
As a chronic refractory wound, diabetic ulcer healing also involves cell proliferation, angiogenesis and other processes. Several studies have explored the molecular mechanism of low-intensity ultrasound combined with microbubble therapy, namely, the enhanced effect of local blood flow is related to the increased synthesis of local vasodilators Nitric oxide (NO) and prostaglandin ( 13 ), while the production of ATPase makes cell proliferation and metabolism more active ( 6 ). At the same time, this method can increase vascular endothelial growth factor (VEGF) and other growth factors and promote angiogenesis ( 14 ). Hypoxia-inducible factor-α (HIF-α) and the activation of immune pathway are also involved in this process ( 15 , 16 ). In this case, the wounds of the 2 patients were gradually and slowly healed after short treatment. The timing and molecular mechanism of initiating active wound repair and continuing to heal need to be further studied.
Diagnostic low-intensity ultrasound was selected in this study primarily for the safety of human trials. Due to the strict FDA restrictions ( 17 ), the acoustic intensity of diagnostic ultrasound is constrained within an admissible range (0.05-0.5 W/cm 2 ) in the form of low energy. As the cavitation nuclei, the microbubbles can reduce the cavitation threshold and enhance the cavitation effect ( 18 , 19 ). Moreover, the visualization advantage of diagnostic low-intensity ultrasound enables it to observe the whole process of microbubble perfusion and rupture. In the experiment, it was observed that the muscular tissue perfusion microbubbles disappeared after a flash operation. From this, it can be speculated that the existing parameter Settings caused Sonoporation in the treatment, and the physical effects such as shear waves and micro jets may promote the occurrence of healing events. High parameters of MI (0.89 and 0.86), sound power (79%) and Frame (50) were set to improve the duty cycle of treatment pulses, and Flash mode was selected to promote the occurrence of cavitation through breaking microbubbles. The possibility of cavitation induced by commercial equipment was also confirmed by Lindar (6) and Kitoplis ( 10 ).
The importance of this study may be reflected in the following aspects: firstly, it was a real world clinical study in humans, and secondly, the therapeutic equipments and microbubbles used are commercial products, which could make this technology promising for clinical promotion and could provide a new non-invasive method and idea for the treatment of diabetic ulcers. The limitations of this study mainly focus on the small sample size, the interpretation of the results needs to be cautious, and more samples need to be accumulated to further verify the treatment effect.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of The General Hospital of Western Theater Command of PLA. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JT, RW and ZC: conception and design of the work. XZ and LP: data collection. XZ, YC and RW: Image analysis and interpretation, manuscript writing, and critical revision of the article. ZC: approval of the final version of the article.
This work received grants from Key Research and Development Program of Science and Technology Department of Sichuan Province (2020YFS0122), and Project of Hospital management of General Hospital of Western Theater Command of PLA (2019ZY11).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med (2017) 376:2367–75. doi: 10.1056/NEJMra1615439
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Costa RHR, Cardoso NA, Procopio RJ, Navarro TP, Dardik A, de Loiola Cisneros L. Diabetic foot ulcer carries high amputation and mortality rates, particularly in the presence of advanced age, peripheral artery disease and anemia. Diabetes Metab Synd (2017) 11 Suppl 2:S583–S7. doi: 10.1016/j.dsx.2017.04.008
CrossRef Full Text | Google Scholar
3. Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K. Microvascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol (2020) 18:117–24. doi: 10.2174/1570161117666190502103733
4. Chapouly C, Yao Q, Vandierdonck S, Larrieu-Lahargue F, Mariani JN, Gadeau A-P, et al. Impaired hedgehog signalling-induced endothelial dysfunction is sufficient to induce neuropathy: implication in diabetes. Cardiovasc Res (2016) 109:217–27. doi: 10.1093/cvr/cvv263
5. Hogan B, Shen Z, Zhang H, Misbah C, Barakat AI. Shear stress in the microvasculature: influence of red blood cell morphology and endothelial wall undulation. Biomech Model Mechan (2019) 18:1095–109. doi: 10.1007/s10237-019-01130-8
6. Belcik JT, Davidson BP, Xie A, Wu MD, Yadava M, Qi Y, et al. Augmentation of muscle blood flow by ultrasound cavitation is mediated by ATP and purinergic signaling. Circulation (2017) 135:1240–52. doi: 10.1161/CIRCULATIONAHA.116.024826
7. Chen L, Zheng Q, Chen X, Wang J, Wang L. Low-frequency ultrasound enhances vascular endothelial growth factor expression, thereby promoting the wound healing in diabetic rats. Exp Ther Med (2019) 18:4040–8. doi: 10.3892/etm.2019.8051
8. Vander Horst MA, Raeman CH, Dalecki D, Hocking DC. Time- and dose-dependent effects of pulsed ultrasound on dermal repair in diabetic mice. Ultrasound Med Biol (2021) 47:1054–66. doi: 10.1016/j.ultrasmedbio.2020.12.024
9. Wakabayashi N, Sakai A, Takada H, Hoshi T, Sano H, Ichinose S, et al. Noncontact phased-array ultrasound facilitates acute wound healing in mice. Plast Reconstr Surg (2020) 145:348E–59E. doi: 10.1097/PRS.0000000000006481
10. Kotopoulis S, Dimcevski G, Gilja OH, Hoem D, Postema M. Treatment of human pancreatic cancer using combined ultrasound, microbubbles, and gemcitabine: A clinical case study. Med Phys (2013) 40(7):072902. doi: 10.1118/1.4808149
11. Chen X, Qiao X, Cuo YI, Liu Y, Zhu Q, Rong Y, et al. Microbubble mediated diagnostic ultrasound enhance blood perfusion of breast cancer. J Clin Ultrasound Med (2018) 020:82–5. doi: 10.16245/j.cnki.issn1008-6978.2018.02.004
12. Qiao X, Chen Z, Yi C, Gao W, Gao S, Liu Z. Vascular effect of rabbit VX2 tumor induced by diagnostic ultrasound with microbubbles. J Clin Ultrasound Med (2017) 019:217–21. doi: 10.16245/j.cnki.issn1008-6978.2017.04.001
13. Suchkova VN, Baggs RB, Sahni SK, Francis CW. Ultrasound improves tissue perfusion in ischemic tissue through a nitric oxide dependent mechanism. Thromb Haemostasis (2002) 88:865–70. doi: 10.1055/s-0037-1613315
14. Deng L-D, Qi L, Suo Q, Wu S-J, Mamtilahun M, Shi R-B, et al. Transcranial focused ultrasound stimulation reduces vasogenic edema after middle cerebral artery occlusion in mice. Neural Regener Res (2022) 17:2058–63. doi: 10.4103/1673-5374.335158
15. Maan ZN, Januszyk M, Rennert RC, Duscher D, Rodrigues M, Fujiwara T, et al. Noncontact, low-frequency ultrasound therapy enhances neovascularization and wound healing in diabetic mice. Plast Reconstr Surg (2014) 134:402E–11E. doi: 10.1097/PRS.0000000000000467
16. Zhang Z-C, Yang Y-L, Li B, Hu X-C, Xu S, Wang F, et al. Low-intensity pulsed ultrasound promotes spinal fusion by regulating macrophage polarization. BioMed Pharmacother (2019) 120:109499. doi: 10.1016/j.biopha.2019.109499
17. U.S. Department of Health and Human Services, Information for Manufacturers Seeking Marketing Clearance of Diagnostic Ultrasound Systems and Transducers. Food and Drug Administration (2008).
Google Scholar
18. Khanna A, Nelmes RT, Gougoulias N, Maffulli N, Gray J. The effects of LIPUS on soft-tissue healing: a review of literature. Bbrit Med Bull (2009) 89:169–82. doi: 10.1093/bmb/ldn040
19. Guo X, Li Q, Zhang Z, Zhang D, Tu J. Investigation on the inertial cavitation threshold and shell properties of commercialized ultrasound contrast agent microbubbles. J Acoust Soc Am (2013) 134:1622–31. doi: 10.1121/1.4812887
Keywords: diabetic foot ulcer, diagnostic ultrasound, microbubble, ultrasound treatment, case report
Citation: Zhang X, Cheng Y, Pei L, Tao J, Wang R and Chen Z (2022) Case report: Successful treatment of human diabetic foot ulcer using low-intensity diagnostic ultrasound combined with microbubbles: Two cases. Front. Endocrinol. 13:1046896. doi: 10.3389/fendo.2022.1046896
Received: 17 September 2022; Accepted: 09 November 2022; Published: 25 November 2022.
Reviewed by:
Copyright © 2022 Zhang, Cheng, Pei, Tao, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhong Chen, [email protected] ; Rui Wang, [email protected]
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Open access
- Published: 06 September 2023
Off-loading and compression therapy strategies to treat diabetic foot ulcers complicated by lower limb oedema: a scoping review
- Justine Tansley ORCID: orcid.org/0000-0003-1160-4275 1 , 2 ,
- Richard Collings 1 , 2 ,
- Jennifer Williams 1 , 2 &
- Joanne Paton 2
Journal of Foot and Ankle Research volume 16 , Article number: 56 ( 2023 ) Cite this article
2561 Accesses
2 Citations
5 Altmetric
Metrics details
Lower limb oedema is a common co-morbidity in those with diabetes and foot ulceration and is linked with increased amputation risk. There is no current guidance for the treatment of concurrent diabetic foot ulcers and lower limb oedema, leading to uncertainty around the safety and efficacy of combination approaches incorporating offloading and compression therapies.
To determine indications and contraindications for such strategies and identify any other supplementary treatment approaches, a scoping review was undertaken to map the evidence relating to off-loading and compression therapy strategies to treat both diabetic foot ulcers and lower limb oedema in combination.
Following the Joanna Briggs Institute (JBI) and PRISMA – Scoping Review (ScR) guidance, this review included published and unpublished literature from inception to April 2022. Literature was sourced using electronic databases including Cochrane Library, PubMed, CINAHL, AMED; websites; professional journals and reference lists of included literature. Eligible literature discussed the management of both diabetic foot ulceration and lower limb oedema and included at least one of the treatment strategies of interest. Data extraction involved recording any suggested off-loading, compression therapy or supplementary treatment strategies and any suggested indications, contraindications and cautions for their use.
Five hundred twenty-two publications were found relating to the management of diabetic foot ulcers with an off-loading strategy or the management of lower limb oedema with compression therapy. 51 publications were eligible for inclusion in the review. The majority of the excluded publications did not discuss the situation where diabetic foot ulceration and lower limb oedema present concurrently.
Conclusions
Most literature, focused on oedema management with compression therapy to conclude that compression therapy should be avoided in the presence of severe peripheral arterial disease. Less literature was found regarding off-loading strategies, but it was recommended that knee-high devices should be used with caution when off-loading diabetic foot ulcers in those with lower limb oedema. Treatment options to manage both conditions concurrently was identified as a research gap. Integrated working between specialist healthcare teams, was the supplementary strategy most frequently recommended. In the absence of a definitive treatment solution, clinicians are encouraged to use clinical reasoning along with support from specialist peers to establish the best, individualised treatment approach for their patients.
Trial registration
Open Science Framework (osf.io/crb78).
Peer Review reports
Introduction
The management of diabetic foot ulcers (DFU) complicated by the effects of lower limb oedema is clinically challenging. Both conditions can be complex requiring a multi-faceted treatment approach. Wound healing is often prolonged in the presence of oedema because it reduces capillary blood flow [ 1 ]. Fluid accumulation in the limbs increases wound exudate levels, raising the risk of infection and further tissue breakdown [ 2 ]. Subsequent increase in limb weight can affect mobility, cause joint and soft tissue pain and elevate the plantar pressure and tissue stress transmitted to the foot ulcer [ 1 ].
Two European prospective cohort studies [ 3 , 4 ], have linked lower limb oedema with an increased risk of amputation in those with a DFU. A further retrospective cohort study found survival rates were poor, following diabetes-related leg amputations [ 5 ]. These studies are widely acknowledged and cited amongst the literature, yet are limited as they only provide an observation of the potential impact that oedema has on the outcomes of DFU. They do not introduce interventions or strategies to manage the two conditions together.
According to International guidelines, DFU often require an ‘off-loading’ intervention to relieve pressure [ 6 ]. The specific nature of an off-loading intervention varies depending on wound location and factors such as ischaemia and infection [ 6 ]. International guidance recommends a non-removable, knee-high off-loading device, such as a total contact cast, as the first-line treatment option to promote wound healing in DFU [ 6 , 7 ]. Physical symptoms produced by lower limb oedema such as increased limb size or volume, wet and leaking skin, leg ulceration and eczematous skin conditions, may prohibit the use of such knee-high off-loading interventions and lead to compromise.
Alternative ankle-high off-loading devices followed by felted foam in combination with appropriately fitting footwear, are suggested as the last treatment resort [ 6 , 7 ]. These may appear more suitable for a person with symptoms of lower limb oedema, but the evidence suggests that they are not as effective in treating DFU [ 7 ].
The benefit of oedema management to improve DFU outcomes is widely acknowledged [ 1 , 2 ], yet it is not routinely considered as part of the standard multi-faceted approach to DFU management, where treatment of complications arising from peripheral arterial disease, neuropathy, infection and foot deformities are a priority [ 1 , 2 ]. Compression therapy is considered a primary intervention in the management of lower limb oedema [ 8 ] and supported by a strong evidence base of randomised controlled trials and systematic reviews [ 9 ].
However, clinicians could be unsure how to overcome the practical challenges for the use of compression therapy when a DFU is also being managed, as this remains an area which is poorly understood [ 2 ], alongside the absence of any definitive guidance for treatment.
A scoping review method was chosen due to the broad nature of the research question and the lack of definitive randomised control trials in the area of DFU management where lower limb oedema is an added complication. This method is best suited to map the evidence base and identify any gaps in the literature [ 10 ] relating to off-loading and compression therapy strategies to manage both diabetic foot ulcers and lower limb oedema in combination.
An initial search for systematic and scoping reviews found five systematic reviews evaluating the effectiveness of various strategies to manage or enhance the healing of DFU, all of which acknowledge lower limb oedema as a risk factor [ 11 , 12 , 13 , 14 , 15 ] and one scoping review exploring the effect of compression bandaging on the healing of DFU [ 16 ]. None examined a multi-morbidity approach to scoping the evidence base specifically focusing on management strategies where diabetic foot ulcers and lower limb oedema co-exist.
The aim of the review was to map any available evidence and literature to determine the off-loading and compression therapy strategies evaluated to treat both DFU and lower limb oedema. To also further understand which strategies are not recommended for this population, identify any other supplementary treatment strategies and determine any gaps in the literature.
Objectives of the scoping review were to establish
Which off-loading strategies can be used to treat DFU for people who also have lower limb oedema?
Which off-loading strategies are not recommended or contraindicated in the treatment of DFU for people who also have lower limb oedema?
Which compression therapy strategies to manage lower limb oedema can be used where a DFU is present?
Which compression therapy strategies are not recommended or contraindicated in the management of lower limb oedema where a DFU is present?
Whether any other supplementary treatment strategies can be identified from the review?
What are the gaps surrounding the strategies to manage DFU and lower limb oedema in combination, in the current literature?
Protocol and registration
A scoping review protocol was developed using the Joanna Brigg’s Institute (JBI) guidance on scoping reviews [ 10 ] and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis – Scoping Review (PRISMA-ScR) checklist [ 17 ]. It is recommended that protocols are registered with research organisations to help avoid the duplication of work and encourage collaborations [ 10 ]. This protocol was prospectively registered with the Open Science Framework on 21/01/2022 available at: https://doi.org/10.17605/OSF.IO/CRB78 (Registration number: osf.io/crb78).
Inclusion criteria
Any information (published or unpublished) relating to DFU management with an off-loading strategy.
Any information (published or unpublished) relating to lower limb oedema management with a compression therapy strategy.
Any information (published or unpublished) relating to the management of a DFU and lower limb oedema, where both conditions present together.
Literature in the context of improved outcomes: wound healing, amputation rates, infection rates, quality of life or care delivery;
Information available in the English language (for feasibility reasons).
Information inclusive of any geographical regions, cultural backgrounds, gender, research methods, care setting, care provider or publication date.
Information sources
This scoping review included both published and unpublished literature. Published sources included: electronic databases such as, Cochrane, PubMed, CINAHL; Professional journals; National and International organisations and charities responsible for publishing guidance. Unpublished sources included: conference abstracts; patient and clinician advice websites; commercially available trials and information.
Search and screening strategy
This scoping review followed the JBI’s recommended search strategy consisting of three steps [ 10 ]. (Searching took place between 10th January – 1 st April 2022). Two key databases (PUBMED, CINAHL) were used in a preliminary search by the first reviewer (JT) and assisted in the refining of search terms with the support of an information specialist. A second search was performed across all the information sources using the refined set of search terms, with consideration being given to alternative spellings of key words (oedema/edema/odema). A third search examined any reference lists, to identify any further literature of use. A full list of search terms can be viewed in Appendix .
The title and abstract was independently screened by two reviewers (JT, JW) on all of the literature found. A pilot screening took place to ensure both reviewers were clear and consistent with the eligibility criteria before the principle screening. Once eligible literature was determined, full text screening was carried out by the first reviewer (JT).
Data charting and data items
A table was prepared in Microsoft Excel, adapted from a JBI template [ 10 ], to record findings from the data extraction exercise. This was used as a prompt to record any relevant findings from each piece of literature such as the treatment strategy, methods, outcomes and any other key findings. A chart for mapping the literature was developed in Microsoft Excel, linked to the objectives and eligibility criteria of the scoping review, which followed the required reporting items for scoping reviews [ 17 ]. Its purpose was to assist in identifying any relevant concepts in context with the scoping review and identify any gaps in the literature.
Appraisal of literature
Although scoping reviews are not intended to synthesise results or require a risk of bias assessment unlike a systematic review [ 10 ], the literature was mapped against the Alper & Haynes (2016) integrated ‘6S’ levels of organisation of evidence pyramid model [ 18 ] to give an impression of the quality of the available literature and its validity to everyday clinical practice.
A total of 522 pieces of literature were found from all searches. Fifty-one pieces of information were included in the final scoping review as detailed in Table 1 . All of the included information addressed both conditions and included at least one of the management strategies of interest. Some of the literature discussed more than one strategy. A summary of the searching and screening process is displayed in the PRISMA flow diagram in Fig. 1 . Publications that did not discuss the situation where diabetic foot ulceration and lower limb oedema present concurrently, was the most common reason for exclusion at both the title and abstract screening ( n = 378, 88%) and full text screening ( n = 24, 59%) stages.
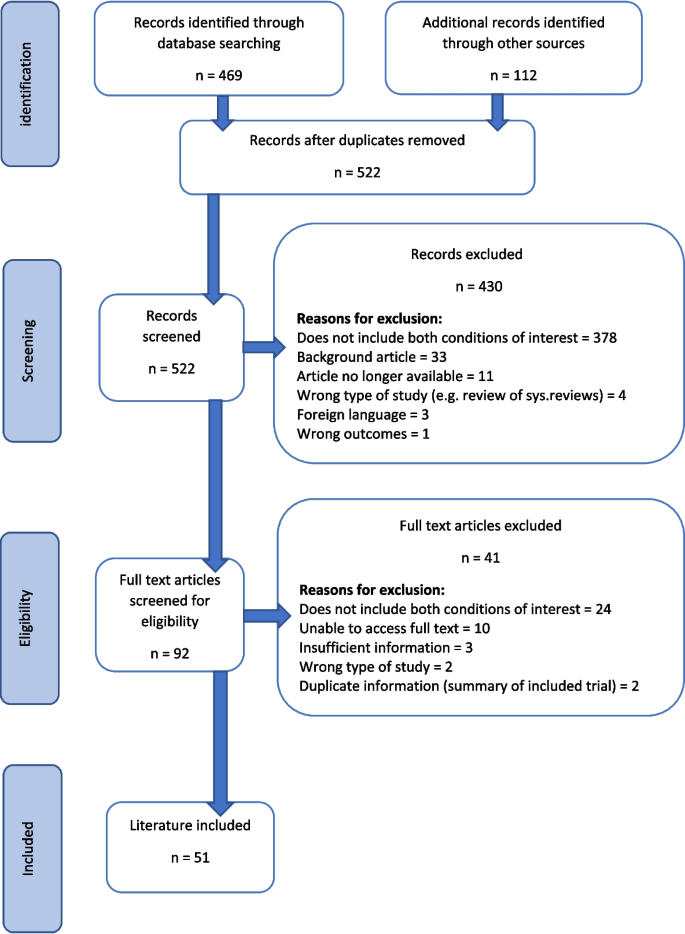
PRISMA flow diagram for the scoping review process [ 10 , 17 ]
Literature characteristics
The included literature spanned a date range of 24 years (1998 – 2022). It was produced from 13 different countries with the UK ( n = 21, 41%) and USA ( n = 10, 20%) being the most prevalent. 44 pieces of literature came from a published source (86%) and seven from unpublished sources (14%). Literature considered to be higher in quality such as evidence-based summaries and guidance, evidence synthesis and research studies [ 18 ] were fewer in numbers ( n = 21, 41%). Foundational resources and unpublished literature which is considered to be lower in quality [ 18 ], was higher in numbers ( n = 30, 59%). Details for evidence type can be viewed in Table 1 .
The majority of the included literature related to the use of compression therapy as a strategy to manage lower limb oedema where a DFU is present ( n = 24, 51%). There was less information available regarding off-loading strategies ( n = 13, 25%). Only three pieces of literature discussed the use of both an off-loading and compression therapy strategy simultaneously (6%). Nine pieces of literature solely focused on a supplementary strategy (18%), although 16 supplementary strategies were identified in total across all of the included literature. Details for these results can be viewed in Table 1 .
Off-loading strategies recommended or contraindicated in the treatment of a DFU for people who also have lower limb oedema
The off-loading strategies to treat a DFU in those with lower limb oedema, mapped against the review objectives, can be viewed in Table 2 . Total contact casting in the presence of lower limb oedema was most frequently discussed in the literature ( n = 5) [ 1 , 41 , 42 , 43 , 44 ]. This type of cast was described to primarily treat a diabetic foot ulcer by immobilising the foot and ankle and off-loading pressure from the wound area. However, appropriate use where lower limb oedema is present appeared uncertain. One retrospective cohort study [ 41 ], found that oedema was a contributory factor to adverse events in those receiving treatment for a DFU, such as the development of a new wound, infection, pain or discomfort requiring cast removal. The study found the patient population most prone to complication was those with "neuropathy and limb volume fluctuation due to both venous insufficiency and vasomotor lymphoedema”. Yet another piece of literature also suggests that the firm outer casing of the cast could be used to prevent or reduce oedema [ 42 ], although the author acknowledges that their suggestion is anecdotal. Peripheral neuropathy [ 43 ], osteomyelitis [ 2 , 41 , 44 ], soft tissue infection/cellulitis [ 2 , 41 , 43 , 44 ] and varicose veins [ 44 ] were suggested contraindications across all of the literature.
Six pieces of literature discussed the use of removable walking casts or boots as detailed in Table 1 . All of the literature agrees the primary purpose is to off-load pressure from the wound area [ 27 , 45 , 46 , 47 , 48 , 49 ]. Four publications, discussed knee-high devices, of which one author advocates using the ridged nature of a knee-high device to act in reducing limb volume [ 45 ]. Yet other information advises that such a device should protect the limb from further damage by accommodating oedema rather than reducing it [ 46 , 47 ]. The remaining two publications discussed the use of an ankle-high device. One case study [ 49 ] describes how a removable ankle boot was used to allow for the use of compression bandages. However, specific indications and contraindications or adverse effects were not reported.
A back-slab casting technique [ 49 ] and a Scotchcast™ boot [ 28 ], were described in two pieces of literature to treat DFU. However, there was insufficient information to determine whether these strategies could be used with oedema management strategies.
The remaining two strategies found, included an off-loading shoe [ 50 ], which is intended to be used with off-loading insoles and a heel reliever [ 51 ], used to treat a DFU occurring at the heel when a person is in the prone position. Both devices state they are designed to accommodate oedema, but not suitable for those with associated complications of oedema such as leg ulcers or lymphorrhoea.
Compression therapy strategies recommended or contraindicated to manage lower limb oedema where a DFU is present
Compression therapy strategies to manage lower limb oedema where a diabetic foot ulcer is present, mapped against the review objectives, can be viewed in Table 3 . This scoping review found eleven pieces of information across all of the literature, suggesting that compression bandaging was an effective way to reduce and manage oedema (as detailed in Table 1 and 2 ), which additionally could have beneficial effects on the healing of DFU [ 16 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 29 , 40 ]. One scoping review [ 16 ] was found which explored the effect of compression bandaging on the healing of DFU. Compression bandaging was deemed to be safe in those without severe arterial compromise. Several case studies were found [ 19 , 21 , 22 , 24 , 26 ], all describing challenging examples where DFU management was complicated by lower limb oedema. A change was made to usual care, by introducing compression bandaging to reduce oedema and achieving a more positive outcome. Two further case studies [ 27 , 49 ] also introduced an offloading intervention to treat plantar DFU in addition to compression therapy. All of the literature reported a positive change to DFU outcomes but none gave suggestions for contraindications or reports of adverse incidence.
The review found 10 pieces of information across all of the literature which suggests that compression hosiery or wrap systems could be useful in managing lower limb oedema where a DFU is present [ 1 , 20 , 27 , 28 , 30 , 31 , 32 , 33 , 34 , 35 ] (Tables 1 and 3 ). A prospective study [ 30 ], and a 12-week, double blind, randomised controlled trial [ 31 ] were found, whose studies used participants with diabetes, with and without mild to moderate peripheral arterial disease, to test the safety of compression hosiery. Both studies also reported that compression hosiery was safe in the absence of severe peripheral arterial disease. However, participants with larger wounds, copious amounts of exudate and infection were excluded, suggesting their use was not considered suitable for larger, more complex wounds.
The use of pneumatic compression systems to manage lower limb oedema and improve healing of DFU was found in the literature and further suggests that it may be used even where severe peripheral arterial disease or non-revascularisable conditions are present [ 34 , 36 , 37 , 38 , 39 ]. However, two publications cited supporting studies which acknowledge that their sample sizes were small and studies were of low methodological quality [ 38 , 39 ] .
Supplementary strategies identified from the review
The identified supplementary strategies to manage a DFU and lower limb oedema where both conditions present together, and mapped against the review objectives, can be viewed in Table 4 . A total of 16 supplementary strategies were identified across all of the included literature (Table 4 ).
Integrated working, where multiple conditions such as DFU and oedema management may require input from multiple teams, was the most frequently mentioned supplementary strategy( n = 5) [ 20 , 25 , 52 , 53 , 54 ] and was one of the suggestions which could be applied to any clinical situation. However, this particular suggestion, despite its inclusion in two national guidance documents [ 52 , 53 ], is referenced as based on expert opinion rather than scientific study. A similar suggestion is made by two best practice statements [ 54 , 55 ], also based on expert opinion, which recommend that treatment plans should be specifically tailored to meet the individual needs of patient to maximise treatment quality. A clinical review piece [ 56 ] and a conference abstract [ 57 ] were found discussing the use of specifically designed wound and limb assessment and triage tools. Both tools acknowledged lower limb oedema as a risk factor to diabetic foot ulcers and suggest they could be used as a prompt to encourage oedema management as part of DFU treatment, further encouraging tailored treatment plans and integrated working.
Other suggested supplementary strategies included: Patient education [ 58 ], leg elevation [ 2 , 20 , 58 , 59 ], elbow crutches [ 45 , 49 ], exercise [ 25 , 27 , 60 ], weight control [ 20 , 60 ], manual lymphatic drainage [ 1 , 25 ], bed rest [ 27 ], skin care [ 25 ], neuromuscular taping [ 61 ], pharmacological [ 60 ] and surgical options [ 62 ]. The evidence to support these supplementary interventions came from foundational sources including case studies, literature reviews and expert opinion pieces which are considered to be of lower evidential quality [ 18 ].
A scoping review was carried out which aimed to establish what available off-loading and compression therapy strategies exist to manage a DFU complicated by the effects of lower limb oedema. Information from 51 pieces of literature were studied. The included studies used various outcomes to assess effectiveness and the overall level and quality of evidence was variable, making interpretation of the results difficult.
Off-loading strategies
International guidance [ 6 , 7 ] recommends that a non-removable knee-high cast, such as a total contact cast (TCC), is used as a first-line treatment to off-load a DFU, unless contraindicated. This scoping review found one retrospective cohort study which suggests that lower limb oedema may be one of these contraindications [ 41 ]. The study suggests that a TCC is not suitable for those with a DFU and lower limb oedema as an increased number of adverse events was reported in this population. It was agreed that such devices were primarily intended to assist with DFU healing, yet there were opposing arguments about their use in the presence of oedema and associated complications. Definitive direction regarding the indications and contraindications for the use of a TCC in these circumstances was lacking from the evidence.
Current guidance also recommends that a knee-high walking cast may be used as a second-line alternative if a non-removable TCC is not tolerated [ 6 , 7 ]. The literature found by the review was conflicting. Some of the literature suggests that a removable knee-high walking cast should accommodate lower limb oedema for limb protection [ 46 , 47 ], yet other literature supports the use of a removable pneumatic walker cast, to off-load a foot wound and reduce oedema [ 45 ]. However, both suggestions were not supported by scientific studies or other forms of evidence. There was a lack of information regarding the use of knee-high removable casts/walkers to treat a DFU where lower limb oedema was present and no discussion was found concerning appropriate use or contraindications in these circumstances.
An ankle-high removable cast is a third-line recommendation, if a knee-high cast is not tolerated or contraindicated [ 6 , 7 ]. The International Working Group for the Diabetic Foot, acknowledge this recommendation in their guidance is not supported by high quality evidence [ 6 ]. The literature found by the review, suggests that an ankle-high design is intended to allow for treatment of a leg condition [ 27 , 48 ], yet it is difficult to make a definite conclusion as to the suitability of this strategy to treat a DFU in the presence of lower limb oedema. No scientific studies were found demonstrating that these off-loading devices could be safely and effectively used in combination with a leg treatment such as compression therapy.
Two further strategies were found which are not included in any current guidance. They included: The use of a back-slab style cast [ 49 ], to off-load a diabetic foot ulcer and accommodate any fluctuations in lower limb oedema; a heel off-loading device [ 51 ] designed to relieve pressure from a heel wound when a person is lying prone, which may accommodate leg swelling but it is not suitable if leg wounds or exudate are present. Both strategies were not supported by scientific studies or other forms of high-level evidence.
Compression therapy strategies
Although there is no current guidance for the use of compression therapy to manage lower limb oedema in the presence of a DFU, benefits for its use are acknowledged in the literature [ 16 ]. This scoping review found that full-strength multi-layer bandaging may be used in those without arterial compromise; reduced-strength bandaging may be used in those with reduced arterial blood supply; and a wound was unlikely to heal if there was severe arterial compromise as compression is likely to further reduce blood flow [ 16 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 29 , 40 ]. Several case studies [ 21 , 22 , 24 , 26 , 27 , 37 , 42 , 49 ] were found all sharing successful practice where DFU management was complicated by lower limb oedema. All of the case studies introduced compression bandaging to promote wound healing. However, reports of failed or ineffective cases and their circumstances were not found, leaving unanswered questions about the true safety and effectiveness of compression bandaging in these circumstances.
This review found literature which suggests that compression hosiery could be a useful way to manage lower limb oedema where a DFU is present [ 1 , 20 , 27 , 28 , 30 , 31 , 32 , 33 , 34 , 35 ]. A prospective study [ 30 ] and a 12-week, double blind, randomised controlled trial [ 31 ], used participants with diabetes, with or without mild to moderate peripheral arterial disease, to test the safety of compression hosiery. Both studies reported that there was no effect on arterial blood supply when hosiery was worn and after removal. Participants with DFU were included in the studies, but the effect on which, was not included as an outcome measure. It is therefore unknown the effect compression hosiery has on the outcomes of DFUs. Participants with large wounds, copious amounts of exudate and infection were excluded, which suggests this strategy may not be appropriate for those with more severe complex wounds.
This review found literature which suggests the use of pneumatic compression to manage lower limb oedema where a diabetic foot ulcer was also present [ 34 , 36 , 37 , 38 , 39 ]. Wound healing and prevention of major amputation were the main outcomes of interest. The majority of the literature agreed that pneumatic compression could be used to promote healing in wounds of any aetiology, including in those with severe peripheral arterial disease where re-vascularisation is not possible. However, the literature acknowledges the supporting evidence to be of low methodological quality.
Supplementary strategies
This scoping review found 16 supplementary strategies to manage a DFU and lower limb oedema where both conditions present together. Integrated working [ 20 , 25 , 52 , 53 , 54 ], patient specific treatment plans [ 54 , 55 ] and the use of wound and leg assessment tools [ 56 , 57 ] was popular in expert opinion. The rationale for these three strategies was they could be applied to any clinical situation including where complex co-morbidities exist which impact the lower limb, used to improve the quality of treatment planning and subsequent care and outcomes. However, all of the supplementary strategies found by this scoping review, lacked a scientific basis to support their use in a combination management approach of a DFU and lower limb oedema.
Implications for practice and future research
This scoping review offers some insight into the available strategies to treat both a DFU and lower limb oedema when they present together and the evidence to support their safe and effective use. It would appear that more scientific evidence is required to determine which off-loading strategy would be the most suitable for use where lower limb oedema is present or if a concurrent oedema management strategy were being considered. Clear guidance on the indications and contraindications for the use of such off-loading strategies in these circumstances would also be welcomed. To further understand whether compression bandaging or hosiery is a suitable strategy to manage DFU complicated by the effects of lower limb oedema, more scientific evidence is required investigating the effect compression therapy has on DFU outcomes such as wound healing, infection rates and amputation rates. Further scientific evidence is needed to support the suggestions that integrated working, tailored treatment plans and wound assessment tools can be used as a strategy to improve the outcomes of DFU complicated by the effects of lower limb oedema.
Despite the review being unable to give definitive off-loading and compression therapy treatment solutions, clinicians should still strive to provide the best treatment strategy to manage a DFU where lower limb oedema is also a complicating feature. Whilst considering the information found from this review, clinicians should use their clinical reasoning skills to contemplate: the physiological differences and complications presenting in each individual patient; the purpose and intended outcome of treatment; whilst encouraging collaborative working with specialist teams, to find the most suitable treatment approach.
Review limitations
The majority of the literature found by this review was published in the UK, followed by other western world countries such as the USA and Australia. This could mean that this scoping review is only applicable and relatable to healthcare in these countries. Furthermore, the literature did not consider different racial, ethnic and cultural behaviours and beliefs. This scoping review only included literature which was available in the English language for feasibility reasons. It is known that three pieces of literature had to be excluded at the screening stage as only the abstract was translated into English but not the full text. It is possible that other available literature may have been excluded at the search stage if the abstract was not in English.
The review found that the literature relating to oedema management with compression therapy was not explicit in describing the location or predominating aetiology of concurrently presenting DFU. Likewise, although the off-loading devices discussed in the review were clear their purpose was to relieve pressure from a plantar wound, further information about off-loading wounds at other locations of the foot, where lower limb oedema was a complication, was not found. This identified gap in the literature makes it difficult for the review to make suggestions on the management strategies relating to specific DFU complexities or locations on the foot, when lower limb oedema is an added complication.
This scoping review discovered that lower limb oedema and diabetic foot ulceration was recognised as a common challenge. However, there is insufficient evidence to suggest definitively which off-loading strategies may be used to treat a diabetic foot ulcer complicated by the effects of lower limb oedema.
Limited evidence was found to suggest that a total contact cast may be contraindicated in those with a diabetic foot ulcer and lower limb oedema. In addition, the findings from the literature identified that an ankle-high off-loading device in combination with a compression therapy intervention, is an approach with potential that warrants further research and investigation.
This scoping review has found evidence to support the use of compression bandaging to treat lower limb oedema in the presence of a diabetic foot ulcers, but only where severe peripheral arterial disease can first be excluded. Compression garments such as hosiery, may be useful to manage oedema but only when a foot ulcer is not too large or complicated.
Of the sixteen supplementary strategies identified, none were supported by high quality evidence. Expert clinical opinion, most frequently suggested better integrated working between teams, would result in better foot health outcomes for the person with diabetes when both conditions occur together.
Availability of data and materials
The information used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Diabetic foot ulcer(s)
Joanna Brigg’s Institute
Preferred reporting items for systematic reviews and meta-analysis Scoping reviews
Total contact cast
Kanapathy M, Portou M, Tsui J, Richards T. Diabetic foot ulcers in conjunction with lower limb lymphedema: pathophysiology and treatment procedures. Chronic Wound Care Manage Res. 2015;2:129–36.
Article Google Scholar
Ho T, Leigh R, Tsui J. Diabetic foot disease and oedema. Br J Diabetes Vasc Dis. 2012. https://doi.org/10.1177/1474651412472213 .
Apelqvist J, Larsson J, Agardh C. The importance of peripheral pulses, peripheral oedema and local pain for the outcome of diabetic foot ulcers. Diabetes Med. 1990;7(7):590–4.
Article CAS Google Scholar
Gershater M, Löndahl M, Nyberg P, Larsson J, Thörne J, Eneroth M, Apelqvist J. Complexity of factors related to outcome of neuropathic and neuroischaemic/ischaemic diabetic foot ulcers: a cohort study. Diabetologia. 2009;52:398–407. https://doi.org/10.1007/s00125-008-1226-2 .
Article PubMed CAS Google Scholar
Fortington L, Geertzen J, van Netten J, Postema K, Rommers G, Dijkstra P. Short- and long-term mortality rates after a lower limb amputation. Eur J Vasc Endovasc Surg. 2013;46(1):124–31.
The International Working Group for the Diabetic Foot. IWGDF Guideline on off-loading foot ulcers in persons with diabetes. 2019; https://iwgdfguidelines.org/offloading-guideline/ . Accessed 20 Nov 2021.
Bus S, Armstrong D, Gooday C, Jarl G, Caravaggi C, Viswanathan V, Lazzarini P. Guidelines on offloading foot ulcers in persons with diabetes (IWGDF 2019 update). Diab Metab Res Rev. 2020. https://iwgdfguidelines.org/offloading-guideline/ . Accessed 01 Dec 2021.
Bianchi J, Vowden K, Whitaker J. Chronic oedema made easy. Wounds UK. 2012;8(2). https://wounds-uk.com/made-easy . Accessed 21 Nov 2021.
European Wound Management Association. Position document: understanding compression therapy. 2003. https://ewma.conference2web.com/#resources/279417.Accessed 21 Nov 2021.
Peters M, Godfrey C, McInerney P, Munn Z, Tricco A, Khalil H. Chapter 11: Scoping Reviews (2020 version); JBI Manual for Evidence Synthesis. Joanna Briggs Institute; 2020 [online]. https://doi.org/10.46658/JBIMES-20-12 .
Elraiyah T, Prutsky G, Domecq J, Tsapas A, Nabhan M, Frykberg R, Firwana B, Hasan R. A systematic review and meta-analysis of off-loading methods for diabetic foot ulcers. J Vasc Surg. 2016;63(2):59–87.
Game F, Apelqvist J, Attinger C, Hartemann A, Hinchliffe R, Londahl M, Price P, Jeffcoate W (International working group diabetic foot). Effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes: a systematic review. Diab-Met Res Rev. 2016. https://doi.org/10.1002/dmrr.2707 .
Game F, Hinchliffe R, Apelqvist J, Armstrong D, Bakker K, Hartemann A, Londahl M, Price P. A systematic review of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diab-Met Res Rev. 2012;28:119–41.
Google Scholar
Lazzarini P, Jarl G, Gooday C, Viswanathan V, Caravaggi C, Armstrong D, Bus S. Effectiveness of offloading interventions to heal foot ulcers in persons with diabetes: a systematic review. Diab-Met Res Rev. 2020. https://doi.org/10.1002/dmrr.3275 .
Morona J, Buckley E, Jones S, Reddin E, Merlin T. Comparison of the clinical effectiveness of different off-loading devices for the treatment of neuropathic foot ulcers in patients with diabetes: a systematic review and meta-analysis. Diabetes-Metab Res Rev. 2013. https://doi.org/10.1002/dmrr.2386 .
Article PubMed Google Scholar
Burhan A, Arofiati F. The effect of compression bandages on the healing of diabetic foot ulcers: a scoping review. In: Proceedings of the 4 th International Conference on Sustainable Innovation 2020 – Health Science and Nursing. 2020;33, p. 571–574.
Page M, McKenzie J, Bossuyt P, Boutron I, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2020;372(71). https://doi.org/10.1136/bmj.n71 .
Alper B; Haynes R. EBHC pyramid 5.0 for accessing pre-appraised evidence and guidance. Br Med J: Evid Based Med. 2016. https://doi.org/10.1136/ebmed-2016-110401 .
Angirasa A, Willrich A, Cooper B, Stuck R. Combining bioengineered human dermal replacement and multilayered compression dressings to manage ulcers in a person with diabetes mellitus. A case study. Osteotomy Wound Manage. 2006;52(5):60–64.
Atkin L, Tansley J, Stephenson J. Diabetic foot ulcers: The impact of oedema. Wounds UK. 2018;14(1):41–8.
Boulton Z, Price J. Oedema management in a diabetic patient with foot ulceration and peripheral vascular disease: a case study. Diabetic Foot J. 2016;19:38–42.
Bowering, C. Use of layered compression bandages in diabetic patients. Experience in patients with lower leg ulceration, peripheral edema and features of venous and arterial disease. Adv Wound Care J Prev Heal. 1998;11(3):129–135.
Calianno C, Holton S. Fighting the triple threat of lower extremity ulcers. Nursing. 2007;37(3):57–63.
Chadwick P. Lymphoedema bandaging: the treatment of a patient with a chronic diabetic foot ulcer due to venous insufficiency. Wounds UK. 2006;2(2):84–5.
McIntosh C, Green T. An overview of lower limb lymphoedema and diabetes. J Lymphoedema. 2009;4(1):49–58.
Probst A. Fast, non-invasive hyperspectral imaging tool for the diagnosis and management of complex foot and leg ulcers - part 1. Diabetic Foot J. 2020;23(2):50–4.
Glynn M. Managing lymphoedema in a diabetic foot clinic. Podiatrist. 2021;24(5):45–8.
Edmonds M. A natural history and framework for managing diabetic foot ulcers. Br J Nurs (Tissue Viability Suppl). 2008;17(11):s20–9.
Medi UK. Compression products: compression hosiery. 2022. https://www.mediuk.co.uk/ .
Rother U, Grussler A, Griesbach C, Almasi-Sperling V, Lang W, Meyer A. Safety of medical compression stockings in patients with diabetes mellitus or peripheral arterial disease. BMJ Open Diabetes Res Care. 2020;8.e001316. https://doi.org/10.1136/bmjdrc-2020-001316 .
Wu S, Crews R, Skratsky M, Overstreet J, Yalla S, Winder M, Ortiz J, Andersen C. Control of lower extremity edema in patients with diabetes: double blinded randomised controlled trial assessing the efficacy of mild compression diabetic socks. Diabetes Res Clin Pract. 2017;27:35–43.
Lohmann & Raucher. ReadyWrap. 2022. https://lohmann-rauscher.co.uk/products/compression-wrap-systems/readywrap .
Medi UK. Compression products: compression wrap systems. 2022. https://www.mediuk.co.uk/ .
Simms K, Ennen K. Lower extremity ulcer management: best practice algorithm. J Clin Nurs. 2010;20:86–93.
Wu S, Crews R, Najafi B, Slone-Rivera N, Minder J, Andersen C. Safety and efficacy of mild compression (18–25 mm/hg) therapy in patients with diabetes and lower extremity oedema. J Diabetes Sci Technol. 2012;6(3):641–7.
Article PubMed PubMed Central Google Scholar
Armstrong D, Nguyen H. Improvement in healing with aggressive edema reduction after debridement of foot infection in persons with diabetes. Arch Surg. 2000;153:1405–9.
Filip J. APWCA case study #2: application of end-diastolic pneumatic compression therapy with the circulator boot. Podiatry Manage. 2007;26(9):149–56.
National Institute for Health and Care Excellence. WoundExpress to manage lower leg wounds; Medtech innovation briefing. 2021; https://nice.org.uk/advice/mib261 . Accessed 05 May 2022.
Oped. Diabetic foot range brochure: Oped vadoplex. 2022; https://oped-uk.com/ . Accessed 05 May 2022.
Lohmann & Raucher. Leg ulcer hosiery kit range: Prescribers guide.2022 https://lohmann-rauscher.co.uk/quicklinks/leg-ulcer-hosiery-kits . Accessed 01 May 2022.
Riopelle A, LeDuc R, Wesolowski M, Schiff A, Pinzur M. Risk of complications with the total contact cast in diabetic foot disorders. Foot Ankle Spec. 2021;14(1):25–31.
Tickner A, Jensen J. TCCs & DAMA in obese patients with lymphedema. Podiatry Manage. 2016;35:77–82.
Naude L, Howard A. Total contact casting: A South-African approach to off-loading the diabetic foot. Wounds International. 2015;6(3):6–11.
Whitelaw S. The total contact cast: controversy in offloading the diabetic foot. Wound Care. 2012;(suppli):s16–20.
Dept of Health (Western Australia). Offloading the high-risk foot. 2013; https://wa.gov.au/ . Accessed 01 Apr 2022.
Oped. VACOcast diabetic: User Instructions. 2022; https://oped-uk.com/ . Accessed 05 May 2022.
Oped. VACOped diabetic: User Instructions. 2022; https://oped-uk.com/ . Accessed 05 May 2022.
Oped. VACOpedes diabetic: User Instructions. 2022; https://oped-uk.com/ . Accessed 05 May 2022.
Gurr J. Diabetic neuropathic foot ulcer complicated by lymphoedema: a case study. Australas J Podiatr Med. 2006;40(2):30–4.
Darco Europe. AllRound Shoe: User instructions. 2022. https://darco-europe.com/therapeutic-shoe-allround-shoe.html . Accessed 05 Apr 2022.
Darco Europe. Body Armor Heel Reliever: User Instructions. 2022. https://darco-europe.com/positioning-devices-pressure-off-loading-device-body-amor-heel-reliever.html . Accessed 05 Apr 2022.
National Institute for Health and Care Excellence. Clinical Knowledge Summaries: Leg Ulcer – Venous. 2021. https://cks.nice.org.uk/topics/leg-ulcer-venous/management/venous-leg-ulcers/#managing-oedema . Accessed 21 Nov 2021.
Scottish Intercollagiate Guidelines Network. Management of chronic venous leg ulcers; A national clinical guideline. 2010. https://sign.ac.uk . Accessed 21 Nov 2021.
Wounds UK. Best Practice Statement: Compression hosiery. 2015. https://wounds-uk.com . Accessed 06 May 2022.
Wounds UK. Best Practice Statement: Improving holistic assessment of chronic wounds. 2018. https://wounds-uk.com . Accessed 06 May 2022.
Martinez - De Jesus F, Ibrahim A, Rodriguez-Ramirez N, Zambrano-Loaiza E. (2021) The Latin American Saint Elian Wound Score System (sewss) for the triage of the diabetic foot attack. Cirugía y Cirujanos. 2021;89(5):679–685.
Raji S, Tariq G. Implementing a lean methodology in diabetic foot care management (Conference abstract); 5th congress of the World Union of Healing Societies; Florence; Italy; September 25–29, 2016. J Wound Care. 26(6) Supp 2:313–315.
Hillson R. Lower limb oedema in diabetes. Pract Diabetes. 2017;34(8):266–7.
Park D, Han S, Kim W. Is the foot elevation the optimal position for wound healing of a diabetic foot? J Plastic, Resconstruct Aesthetic Surg. 2010;63:561–4.
Gastaldi G, Pannier F, Roztocil K, Lugil M, Mansilha A, Haller H, Rabe E, Van Rijn M. Chronic venous disease and diabetic microangiopathy: pathophysiology and commonalities. Int Angiol. 2021;40(6):457–69.
Kristianto H, Waluyo A, Gayatri D, Yunir E, Blow D. Neuromuscular taping treatment of diabetic foot: a concept analysis. Clin Ter. 2021;72(3):231–5.
Lin C, Ou K, Chang S. Diabetic foot ulcers combination with lower limb lymphedema treated by staged charles procedure: case report and literature review. Pak J Med Sci. 2013;29(4):1062–106.
Download references
Acknowledgements
National Institute of Health Research—This scoping review was undertaken as part of a National Institute of Health Research funded Pre-Doctoral Clinical Academic Fellowship.
University of Plymouth—The required software and access to electronic databases was available as part of an association with the University of Plymouth.
This scoping review was undertaken as part of a National Institute of Health Research funded Pre-Doctoral Clinical Academic Fellowship (PCAF).
Author information
Authors and affiliations.
Torbay and South Devon NHS Foundation Trust, Torquay, UK
Justine Tansley, Richard Collings & Jennifer Williams
University of Plymouth, Plymouth, UK
Justine Tansley, Richard Collings, Jennifer Williams & Joanne Paton
You can also search for this author in PubMed Google Scholar
Contributions
JT – Primary researcher and author. JW – Second reviewer in screening, selection and review process. RC – Research project supervisor and clinical academic supervisor. Support with design and methodology. JP – Clinical academic supervisor. Support with design and methodology. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Justine Tansley .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Tansley, J., Collings, R., Williams, J. et al. Off-loading and compression therapy strategies to treat diabetic foot ulcers complicated by lower limb oedema: a scoping review. J Foot Ankle Res 16 , 56 (2023). https://doi.org/10.1186/s13047-023-00659-3
Download citation
Received : 10 March 2023
Accepted : 24 August 2023
Published : 06 September 2023
DOI : https://doi.org/10.1186/s13047-023-00659-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diabetic foot ulcer
- Lower limb oedema (edema)
- Off-loading
- Compression therapy
- Wound-healing
- Scoping review
Journal of Foot and Ankle Research
ISSN: 1757-1146
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Subscribe to journal Subscribe
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Failure of Healing in Chronic Diabetic Wounds: A Case Report
Stiehl, James B. MD, MBA
James B. Stiehl, MD, MBA, is an orthopedic surgeon, St Mary’s Hospital, Centralia, Illinois. Acknowledgments: The author thanks Morgan Mulvany, RN, Administrative Director of Odin Care, Odin, Illinois; and Erick Rivas, MD, Vohra Physicians, Chicago, Illinois. The author has disclosed no financial relationships related to this article. Submitted April 1, 2020; accepted in revised form May 13, 2020.
This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND) , where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially without permission from the journal.
Stalled healing in chronic wounds is a challenging problem for providers and remains multifactorial in etiology. Older adults with insulin-dependent diabetes are at very high risk. In this case report, two patients with large nonhealing wounds were considered for treatment with daily jet lavage irrigation in an attempt to remove the inflammatory products of their respective chronic wounds and eliminate the persisting biofilm bacteria. Several attempts were made to reduce treatments to two to three times per week, and negative-pressure wound therapy was initiated in both cases only to see the return of inflammation and necrosis of the wound bed. In both cases, the daily jet lavage irrigation was successful in creating a granulating wound bed that slowly healed over many months. One patient died with an open sacral pressure injury, and the other patient died 4 months after complete healing of a large heel pressure injury. The interesting observation is the necessity of daily high-intensity wound irrigation to correct the chronic infectious process. Diabetic chronic wounds in high-risk older adults are recalcitrant to standard wound treatments, and providers should consider daily jet lavage wound irrigation to deal with this problem.
INTRODUCTION
Healing impairment in chronic diabetic wounds is related to many biologic processes, but a salient factor is the insufficiency of the human immune system to combat bacterial contamination. 1,2 Chronic insulin-dependent diabetes creates vascular disease through molecular changes, deposition of glycation products that stiffen vessels via increased basement membrane permeability, and disrupting basic wound healing processes. 3 Vascular resistance is increased by alteration of vessel diameter, which leads to enhanced tissue ischemia and microvascular sludging at the capillary level. The combined effects of these disorders make patients with other comorbidities fragile and susceptible to infection, amputation, and delayed wound healing. 4,5 Older adults may have malnutrition, cardiovascular disease, and chronic dementia, which add to the risk profile.
This case report considers two such older adults where healing had stalled with standard wound treatments, and the patients were debilitated to the extent that reconstructive surgical procedures were out of the question. From the wound healing literature consensus, the TIME (tissue, infection/inflammation, moisture balance, and edge of wound) paradigm is an important consideration for implementing treatment. The author introduced the Charlson comorbidity index (CCI) to risk stratify the patients in this study. The CCI was introduced in 1987 by Mary Charlson, MD, 6,7 for assessing the effect of chronic disease in patients with cancer by determining 1-year mortality in this setting. Significantly, both cases had a very high CCI, indicating severe chronic underlying disease burden. The other remarkable finding was the efficacy of frequent daily saline irrigations to stabilize both wounds to healthy-appearing granulation tissue. Several attempts were made to accomplish the TIME step-down method only to have necrosis and chronic inflammation return.
This study was sponsored by the author and approved for clinical investigation by the Sterling Investigational Review Board, Atlanta, Georgia (#6606-001). The study used an uncontrolled open-label design, and there were no exclusions to treatment. The study involved high-risk patients for whom treatments had not been successful for a minimum of 3 months. Fourteen patients consented and were treated to assess the safety and efficacy of pressurized jet lavage irrigation for treating problems where wound healing had stalled. For this cohort, the average CCI was 7.6 (CCIs >5 are considered severe). 6,7
Two patients who had treatment extended beyond 26 weeks to a treatment endpoint are the subject of this case report. These patients were of specific interest because of severe diabetic vascular disease, unilateral below-knee amputation, stalled wound healing and chronic wounds that had not responded to 4months’ standard treatment. Both patients provided permission to publish the case details and associated images.
A 65-year-old man was evaluated for treatment of a stage 4 175-cm 2 pressure injury with severe tunneling and exposed bone ( Figure 1 A). He was 6 months postoperation for an intertrochanteric hip fracture of his right leg that had previously undergone a below-the-knee amputation for severe diabetic foot pressure injury. He also had a Wagner stage 3 open pressure injury exposing the metatarsal phalangeal joint of his opposite leg hallux. His comorbidities included stroke, brittle insulin-dependent diabetes, coronary artery disease, peripheral vascular disease, and chronic dementia. His CCI was 10. His serum lymphocyte count was 800 cells/mcL, (normal, >1,500 cells/mcL), and his prealbumin level was 8 mg/dL (normal, >16 mg/dL), indicating infection inflammation and probable increased disease burden. 8,9 The sacral pressure injury developed on his right leg during a 2-week hospitalization for the hip fracture, and he had been discharged from the hospital with negative-pressure wound therapy (NPWT) and daily application of a collagenase for the pressure injury on the sacrum.

After 4 months of treatment, healing of the pressure injury had stalled, and the wound had a gray, smooth appearance without the expected red granulating surface. He had two recent hospitalizations for systemic sepsis managed with IV antibiotics. The negative pressure was difficult to maintain, and the pad was close enough to the anus that the wound bed was often contaminated with feces. The wound produced a large amount of daily slough and extraordinary odor. The treating primary care physician agreed to alternative treatment, and the patient was consented for this study.
Daily treatment was initiated with a handheld pulsatile irrigator delivering 3 L of saline to the wound under “low pressure” (<15 pounds per square inch). 10,11 Treatment was done at the bedside by physical therapists, and effluent was collected in a novel fluid collection bag designed to cover the sacral wound, creating a watertight seal with a wide margin of double-film ostomy tape ( Figure 1 B). Following the recommendations of several prior authors, treatment included irrigation 5 days per week in the outpatient setting. 12–18 To address the wound tunneling, providers used a special long irrigator tip initially designed for femoral canal irrigation in total hip replacements. They also treated the left foot pressure injury with the exposed metatarsal phalangeal joint with 3 L of saline delivered under low-pressure jet lavage irrigation and used a fluid collection bag designed to cover extremity wounds.
Within 10 days, both wounds showed much lower amounts of slough and no odor. Early bedside sharp debridement without anesthetic was required on several occasions for the sacral wound, causing a modest amount of bleeding. 19 The sacral wound was “wet” for the first 10 days, but once granulation tissue was apparent, all minor capillary blood oozing ceased and never recurred. It was later recognized that the patient was on maintenance anticoagulant treatment for his stroke history, peripheral vascular disease, and coronary artery disease. This factor did not cause a complication.
After 5 months of treatment, there was substantial improvement with the sacral wound down to about 60 cm 2 and complete resolution of the tunneling ( Figure 1 C). The sacral wound demonstrated excellent healthy granulation and had no odor, and the patient had no further episodes of systemic sepsis. Reducing irrigation to 3 days per week caused the wound to lose its healthy appearance, so daily jet lavage wound irrigation was resumed.
One month later, the patient had an upper respiratory tract infection that progressed to pneumonia. He returned to the nursing home and resumed treatment with NPWT and collagenase applied to the sacral wound. Within 7days, the wound surface was turning gray, and several small necrotic spots began to appear ( Figure 1 D). Daily jet lavage irrigation was reinstituted, and the necrosis quickly resolved within a week. This exercise was repeated a second time when the patient underwent amputation of the contralateral limb first metatarsal ray and a diverting colostomy ( Figure 1 E). The patient lived 4 more months with a healthy wound, with granulation tissue completely covering the previously exposed 1 area of sacral bone. The patient died of natural causes.
A 70-year-old woman was evaluated for treatment with a 35-cm 2 heel pressure injury ( Figure 2 A). The wound had a dense scab covering 40% of its surface and a moderate amount of slough. The patient had been treated for nearly a year with NPWT and collagenase. The patient had insulin-dependent diabetes and severe peripheral vascular disease, with the contralateral leg amputated from an advanced necrotic foot ulcer. Other comorbidities included severe obstructive pulmonary disease and chronic dementia. Her CCI was 11. Daily jet lavage irrigation was instituted, and within 2 weeks, the fibrotic “black cap” disappeared, with the wound converting to a healthy appearance.

Careful pressure control of the heel and daily irrigation over the next several months significantly improved the wound. The patient had a wound healing relapse at 4 months following 5 days without treatment over a holiday season. Pressure injury may have been involved. The substantial decline in wound appearance quickly improved with daily irrigation. A month later, the patient was admitted with pneumonia and returned to the nursing home with NPWT with daily collagenase to the wound. Over the ensuing week, the wound developed several dark spots and lost the red color of healthy tissue granulation. Daily jet lavage irrigation quickly resolved the adverse outcomes. The wound was completely closed after 10 months of jet lavage irrigation, remaining healed over the next 4 months, after which the patient died ( Figure 2 B).
This case series reveals that there is a level of microvascular disturbance where typical wound treatment with cleansing, moisture management, and even NPWT is not adequate. Also, daily low-pressure irrigation can resolve inflammation and enable angiogenesis. Case 1 had been septic on several occasions requiring hospitalization. His wound was grossly contaminated daily by fecal material, and he eventually had a diverting colostomy. Case 2 had been advised to have a below-the-knee amputation as her wound had not responded to nearly a year of NPWT treatment. Neither patient experienced significant pain during the course of treatment, but case 1 had discomfort during the first few treatments; this was dealt with by using the low-power setting, which produced irrigation at 700 ccs/min versus standard irrigation at 1,200 ccs/min. Case 1 had 169 treatments over a 51-week period, and case 2 had 131 treatments over a 50-week period.
The use of daily jet lavage pulsatile irrigation was significantly less costly than a parallel approach with NPWT, because the method is a surgical procedure that offers a separate CPT code covered by Medicare Part B and Medicaid in the outpatient setting. For case 1, over a 10-month period, the cost was $18,600 for the kits, and the CMS Medicare Part B reimbursement for the kits was $19,225. Case 2 was treated over 12 months at a cost of $14,410 with a CMS Medicare Part B reimbursement of $11,176. The difference relates to the lower reimbursement of the 97597 code used to treat the heel ulcer compared with the 97598 code for the larger wound. The nursing home quoted their cost experience with NPWT systems as averaging $3,000 per month for nonreimbursed dressings. This translates to $30,000 direct costs to the nursing home for case 1 and $36,000 for case 2 that are not covered by a separate CMS code.
The other important consideration is the level of patient comorbidity. A recent hospital study demonstrated that risk factors that lead to lower extremity amputation in patients with diabetes included poor glycemic control, hypertension, hypertriglyceridemia, and infection. 5 The CCI is a recognized tool for assessing the burden of chronic disease and was utilized by the author in all patients of the study cohort. 6,7 The CCI has been upgraded several times in the medical literature with age adjustment and whittling down categories such as peripheral vascular disease where treatments have advanced. There are now a few wound literature citations where this method was a descriptive noncontinuous variable. The CCI or other such standard tools can help guide treatment. For chronic wound infections, blood plasma markers such as transthyretin may be a useful adjunct assessment. Prealbumin (transthyretin), which is produced primarily by the liver and has a baseline blood level of 16 mg/dL from our hospital laboratory, is commonly associated with chronic infection inflammation and malnutrition. C-reactive protein is produced by the liver in acute inflammation at the expense of prealbumin, which is depressed. Depressed prealbumin may be associated inversely with the CCI and is correlated with underlying disease burden. 8,9
Selective mechanical debridement with low-pressure jet lavage irrigation has been well accepted as a method for treating contaminated and infected wounds. 12–18 Pulsatile irrigation does not pose any known risks to outpatients. The Agency for Healthcare Policy and Research reviewed this technology, and the consensus panel set a ceiling pressure at 15 pounds per square inch. The FDA has determined that pulsatile irrigators must be single use and sterilized, but require no premarket approval as they are considered 510(k) exempt. 10,11 The Cochrane Database meta-analysis review would suggest that evidence is generally positive for this method but limited in nature with very few high-evidence randomized controlled trials. 20 That stated, the surgical profession has used this treatment widely for effective cleansing of acute wounds, treatment of infected wounds, and removal of biofilm bacteria in chronic wounds. Mechanical debridement with low-pressure jet lavage irrigation has been indicated as an effective wound bed preparation method prior to advanced treatments under the TIME wound management methodology. 1,10,21 Most states allow midlevel providers such as physician’s assistants, NPs, and physical therapists by statute to independently perform outpatient low-pressure jet lavage therapy and advanced practice wound nurses to provide the treatment under direct physician supervision.
A key problem with wound healing is the biofilm/bacterial bioburden in chronic wounds. Bacteria are highly effective in controlling the local environment, with elevation of factors such as matrix metalloproteinases to extraordinary levels that diminish the ability of the human immune system to control the infection. 2,22 Bjarnsholt et al 2 hypothesized that to reverse this process, providers must remove bacteria and detrimental factors, such as matrix metalloproteinases, from the wound. The medical literature is clear that mechanical debridement is the optimal initial treatment method, but the recent TIME concepts of Schultz et al 1 further advocate using a multimodal approach, such as optimizing overall diabetes care, removing risks of pressure, and optimizing the health of the host. 1,21
For jet lavage, early wound bed preparation for biofilm control is a logical indication for usage. The goal of treatment is to get these wounds stable from the microbiologic viewpoint, that is, less than log 4 to 5 colony-forming units (CFUs)/g, and then resolve the inflammation issues. 10 Alternative treatments could include other mechanical methods such as frequent local sharp debridement, monofilament sponge debrider (Debrisoft, Lohmann & Rauscher, Milwaukee, Wisconsin), low-intensity ultrasound, and typical antiseptic/surfactant solutions. 10,23–25
During this study, the author’s treatment team consistently applied antiseptic solutions each day, and the author tried several, including Prontosan Gel (B. Braun Medical, Bethlehem, Pennsylvania), BlastX Gel (Next Science, Sydney, Australia), and Dakin solution 0.187% (Covenant Medical Services Inc, Maryland Heights, Missouri). A multimodal approach is critical in these cases, and topical antiseptics probably played a role in the outcome. 10
This case report is unique because the TIME step-down approach allowed neither decrease nor discontinuation of the pulsatile irrigators. The human burden of failed or “stalled” treatment should not be minimized. Families of these patients did not want their relative to suffer from a painful wound or wound odor. Because this report comes from an institutional review board study, the families were carefully advised that the treatments, although experimental, were not ill-conceived and offered great potential benefit. They were pleased with what the study demonstrated.
Is there other medical evidence to support the added complexity of daily wound irrigation with a jet lavage procedure? Wolcott et al 26 showed how easily bacteria return to infection-level contamination from baseline at greater than 1 × 10 5 CFUs/g of tissue by 72 hours after surgical debridement. Similarly, Yang et al 27 demonstrated from a porcine skin model that even less virulent species of bacteria than those typically found in a human wound are able to repopulate the wound model to greater than 1× 10 5 CFUs/μL within 48 to 72 hours. Although the clinical situation may not directly mirror these experimental findings, high-risk problems, such as these two cases, are clearly going to behave poorly with standard treatments every 2 to 3 days.
CONCLUSIONS
This case report identified two cases where standard treatments were not effective for advancing wound healing in high-risk patients. Daily jet lavage pulsatile irrigation with 3 L of saline in the outpatient setting was successful in reversing this trend. Significantly, attempts to return to prior treatment or stepping down to less intense daily irrigation caused a recurrence of wound ischemia.
- Cited Here |
- Google Scholar
debridement; diabetes; jet lavage irrigation; negative-pressure wound therapy; TIME paradigm; wound bed preparation
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Venous ulcer assessment and management: using the updated ceap classification..., wound bed preparation 2021, jet lavage irrigation resolves stage 4 pelvic pressure injury undermining, checklist for factors affecting wound healing, clinical order sets for arterial ulcers.
- Diabetes & Primary Care
- Vol:24 | No:05
Interactive case study: Problems with the diabetic foot
- 25 Oct 2022
Share this article + Add to reading list – Remove from reading list ↓ Download pdf
Diabetes & Primary Care ’s series of interactive case studies is aimed at all healthcare professionals in primary and community care who would like to broaden their understanding of diabetes.
Around one in three people with diabetes will develop a foot ulcer within their lifetime. Primary care plays a critical role in identifying problems with the diabetic foot, and in responding rapidly and appropriately to them. The three mini-case studies developed for this issue of the journal take us through the basic considerations of identifying and managing problems with the diabetic foot.
The format uses typical clinical scenarios as tools for learning. Information is provided in short sections, with most ending in a question to answer before moving on to the next section.
Working through the case studies will improve our knowledge and problem-solving skills in diabetes care by encouraging us to make evidence-based decisions in the context of individual cases.
Readers are invited to respond to the questions by typing in your answers. In this way, we are actively involved in the learning process, which is hopefully a much more effective way to learn.
By actively engaging with these case histories, I hope you will feel more confident and empowered to manage such presentations effectively in the future.
Glenda is 62 years old and has had type 2 diabetes for 5 years. She reports an uncomfortable tingling sensation in both her feet that is most troublesome at night, and the feeling of walking on pebbles.
How would you interpret Glenda’s symptoms?
56-year-old Sam has established type 2 diabetes and peripheral neuropathy. An area under his left foot has been weeping, but is not painful. Examination reveals an area of callus and frank ulceration under the first metatarsal head. There is a little bleeding, but no pus discharge or indication of cellulitis. The foot is not deformed, and feels warm with good pulses. A dense sensory peripheral neuropathy is confirmed.
What is your assessment of Sam’s problem?
Proma is a 57-year-old Asian woman with type 2 diabetes. Two years ago she suffered a myocardial infarction. She has a small new ulcer on her right foot, without cellulitis or discharge. The posterior tibial pulse is absent and the dorsalis pedis pulse is weak. The foot is cool, with a dusky tinge to the toes and dystrophic toenails. Peripheral sensation is intact. There is excessive wear on the inside of the lateral aspect of her shoe at the site corresponding to her ulcer.
What is the likely underlying cause of Proma’s foot ulcer?
By working through these interactive case studies, we will consider the following issues and more:
- Causes of peripheral neuropathy.
- Pharmacological management of peripheral neuropathic pain in diabetes.
- Prevention of diabetic foot problems in primary care.
- Neuropathic and ischaemic origins of foot ulcers.
Click here to see the case study.
Editorial: The importance of getting the correct diabetes diagnosis
Interactive case study: mody – a strong family history of diabetes, pcds committee elections (2024): call for candidates, prescribing pearls: a guide to pioglitazone, diabetes distilled: diabetes remission in the real world, hypertension case finding and treatment to target as part of the nhs diabetes eye screening programme, q&a: lipid management – part 3: triglycerides and use of non-statin drugs.

Jane Diggle tackles the challenges of getting the diabetes diagnosis right.

Signs, symptoms, differential diagnosis and management of maturity-onset diabetes of the young (MODY).

How to stand for election to the PCDS Committee.

Understanding pioglitazone: The essential information in one place.
Sign up to all DiabetesontheNet journals
- CPD Learning
- Journal of Diabetes Nursing
- Diabetes Care for Children & Young People
- The Diabetic Foot Journal
- Diabetes Digest
Useful information
- Terms and conditions
- Privacy policy
- Editorial policies and ethics

By clicking ‘Subscribe’, you are agreeing that DiabetesontheNet.com are able to email you periodic newsletters. You may unsubscribe from these at any time. Your info is safe with us and we will never sell or trade your details. For information please review our Privacy Policy .
Are you a healthcare professional? This website is for healthcare professionals only. To continue, please confirm that you are a healthcare professional below.
We use cookies responsibly to ensure that we give you the best experience on our website. If you continue without changing your browser settings, we’ll assume that you are happy to receive all cookies on this website. Read about how we use cookies .
Nursing Case Study for Diabetic Foot Ulcer
Watch More! Unlock the full videos with a FREE trial
Included In This Lesson
Study tools.
Access More! View the full outline and transcript with a FREE trial
Michael is a 15-yr-old male diagnosed with type I diabetes mellitus (DM) last year. He presents to the acute care clinic with a “sore that will not heal” on the bottom of his right foot. He states that he sees an endocrinologist for his DM but has no other health issues to report at this time. He also says that he exercises frequently under the guidance of a dietician and personal trainer consulted through endocrinology and still wrestles for his high school team
His mother is with him and gives consent for treatment. She says, “The pad of his foot has not even been hurting him but when he was pushing off the wrestling mat in practice, he noticed something there.”
What does the nurse suspect may be going on with Michael today? Why?
- A diabetic foot ulcer.
- He is at high risk for foot ulcers (“The lifetime risk of a foot ulcer in patients with diabetes [type 1 or 2] may be as high as 34 percent”)
- He seems to have neuropathy because he does not feel the wound
- He says it will not heal (could be due to many factors especially high blood glucose)
- Assessment should include evaluating and documenting the following:
- Size and depth of the wound
- Color of wound (area and base)
- Look for drainage (exudate)
- Check for loss of sensation (pinprick, touch, pressure) in the lower extremities
- Vascular status (pulse, claudication) in the lower extremities
The nurse notices she cannot feel pedal pulses and the feet seem cool, but not cold. However, the area around the wound is warmer and red. Both feet are WNL in regard to color. Michael denies any cramping or pain in his legs.
What should the nurse do now? How would she document this finding?
- She should get a doppler device prior to proceeding. The pulse may only be weak, not absent. Care should be taken not to document “absent pulse” without first attempting a doppler pulse. Documentation should include whether pulses are palpable or doppler only.
The wound has a slightly foul odor and has a circular “punched out” shape almost like a red based crater. Michael’s mother asks how this could happen.
How should the nurse explain Michael’s risks for this condition?
- Foot problems are a common complication in people with diabetes. Poor glucose control can lead to vascular problems (poor circulation), neuropathy, higher risk for infection (impeding white blood cells from doing their job).
- “Risk factors that can lead to foot wounds in patients with diabetes include loss of protective sensation due to neuropathy, prior ulcers or amputations, foot deformity leading to excess pressure, external trauma, infection, and the effects of chronic ischemia, typically due to peripheral artery disease. Patients with diabetes also have an increased risk for non-healing related to mechanical and cytogenetic factors, as well as a high prevalence of peripheral artery disease”
The provider assesses the patient and advises the staff he has put in orders. He also asks for a set of vital signs (which had not yet been taken).
Why would the provider want vital signs when the patient only has a wound on his foot?
- This is a basic part of an assessment and should never be skipped even if the patient seems healthy. If the patient were to have a change, and no vital signs were taken early on, there would be nothing to compare VS to. Also, if he has an infection, his VS could be the first clue if he were to become septic (signs of septic shock).
BP 110/68 SpO2 98% on Room Air HR 92 bpm and regular Pain 0/10 on 1-10 scale with 10 being highest RR 16 bpm at rest Temp 37.9°C
Michael’s mother asks why the acute care clinic cannot just take care of her son’s foot. She says, “Can’t we just get some antibiotics and go?”
How should the nurse address this issue?
- Foot ulcers in diabetics can be complicated to treat. To prevent complications like worsening infection, possible surgery and/or amputation coordinated care is recommended. A culture of the wound may guide the provider’s choice of antibiotic. Especially since Michael does not seem to feel this wound, it should be addressed more carefully than just a “wound.”
Both patient and mother agree to the prescribed treatments. Before they leave, the provider indicates he wants to talk to them about when to seek a higher level of care.
What does the nurse expect the provider to discuss?
- “Management of diabetic foot infections requires attentive wound management, good nutrition, appropriate antimicrobial therapy, glycemic control, and fluid and electrolyte balance. Although severe infections warrant hospitalization for urgent surgical consultation, antimicrobial administration, and medical stabilization, most mild infections and many moderate infections can be managed in the outpatient setting with close follow-up.
- Several studies have reported improved outcomes with a multidisciplinary approach to diabetic foot infections. This includes involvement of specialists in wound care, infectious diseases, endocrinology, and surgery”
What should the nurse ensure she does in regard to discharge teaching?
- If antibiotics are prescribed, whether now or pending wound culture, the full course must be taken even if everything seems to be improving.
- “It is important to examine your feet every day. This should include looking carefully at all parts of your feet, especially the area between the toes. Look for broken skin, ulcers, blisters, areas of increased warmth or redness, or changes in callus formation; let your health care provider know if you notice any of these changes or have any concerns.”
- Take note of any unusual sensations in the feet and legs, including pain, burning, tingling, or numbness. If you notice these symptoms, keep track of when they happen; whether your feet, ankles, and/or calves are affected; and what measures relieve the symptoms.
Michael says he will follow the prescriptions and follow up as instructed. He also wants to know how to keep this from happening again.
What are some tips the nurse can provide regarding prevention? What about resources to provide?
- Avoid smoking
- Avoid going barefoot, even at home, and especially on hot decks and hot sand
- Test water temperature before stepping into a bath
- Trim toenails to the shape of the toe, and remove sharp edges with a nail file; do not cut cuticles
- Wash in lukewarm water, dry thoroughly (including between the toes), and check feet daily
- Shoes should be snug, but not tight, and customized if feet are misshapen or have ulcers
- Socks should fit and be changed daily
- Resources – for this particular patient, he may like audio-visual education resources. There may also be resources available through his endocrinologist’s office.

View the FULL Outline
When you start a FREE trial you gain access to the full outline as well as:
- SIMCLEX (NCLEX Simulator)
- 6,500+ Practice NCLEX Questions
- 2,000+ HD Videos
- 300+ Nursing Cheatsheets
“Would suggest to all nursing students . . . Guaranteed to ease the stress!”
References:
View the full transcript, nursing case studies.

This nursing case study course is designed to help nursing students build critical thinking. Each case study was written by experienced nurses with first hand knowledge of the “real-world” disease process. To help you increase your nursing clinical judgement (critical thinking), each unfolding nursing case study includes answers laid out by Blooms Taxonomy to help you see that you are progressing to clinical analysis.We encourage you to read the case study and really through the “critical thinking checks” as this is where the real learning occurs. If you get tripped up by a specific question, no worries, just dig into an associated lesson on the topic and reinforce your understanding. In the end, that is what nursing case studies are all about – growing in your clinical judgement.
Nursing Case Studies Introduction
Cardiac nursing case studies.
- 6 Questions
- 7 Questions
- 5 Questions
- 4 Questions
GI/GU Nursing Case Studies
- 2 Questions
- 8 Questions
Obstetrics Nursing Case Studies
Respiratory nursing case studies.
- 10 Questions
Pediatrics Nursing Case Studies
- 3 Questions
- 12 Questions
Neuro Nursing Case Studies
Mental health nursing case studies.
- 9 Questions
Metabolic/Endocrine Nursing Case Studies
Other nursing case studies.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
In-shoe plantar shear stress sensor design, calibration and evaluation for the diabetic foot
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing
Affiliation Department of Mechanical, Aerospace and Civil Engineering (MACE), University of Manchester, Manchester, United Kingdom
Roles Investigation, Methodology, Writing – review & editing
Roles Conceptualization, Data curation, Funding acquisition, Investigation, Resources, Supervision, Validation, Writing – review & editing
Affiliation Medical School, NIHR Exeter BRC, University of Exeter, Exeter, United Kingdom
Roles Investigation, Methodology, Software, Writing – review & editing
Roles Investigation, Software, Writing – review & editing
Roles Resources, Supervision, Writing – review & editing
Affiliation Musculoskeletal Biomechanics and Research in Science and Engineering faculty of Manchester Metropolitan University, Manchester, United Kingdom
Affiliation Manchester University NHS Foundation Trust within the Departments of Diabetes and Vascular Surgery, Manchester, United Kingdom
Roles Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
- Athia H. Haron,
- Lutong Li,
- Jiawei Shuang,
- Chaofan Lin,
- Helen Dawes,
- Maedeh Mansoubi,
- Damian Crosby,
- Garry Massey,
- Neil Reeves,

- Published: September 4, 2024
- https://doi.org/10.1371/journal.pone.0309514
- Peer Review
- Reader Comments
Plantar shear stress may have an important role in the formation of a Diabetic Foot Ulcer, but its measurement is regarded as challenging and has limited research. This paper highlights the importance of anatomical specific shear sensor calibration and presents a feasibility study of a novel shear sensing system which has measured in-shoe shear stress from gait activity on both healthy and diabetic subjects. The sensing insole was based on a strain gauge array embedded in a silicone insole backed with a commercial normal pressure sensor. Sensor calibration factors were investigated using a custom mechanical test rig with indenter to exert both normal and shear forces. Indenter size and location were varied to investigate the importance of both loading area and position on measurement accuracy. The sensing insole, coupled with the calibration procedure, was tested one participant with diabetes and one healthy participant during two sessions of 15 minutes of treadmill walking. Calibration with different indenter areas (from 78.5 mm 2 to 707 mm 2 ) and different positions (up to 40 mm from sensor centre) showed variation in measurements of up to 80% and 90% respectively. Shear sensing results demonstrated high repeatability (>97%) and good accuracy (mean absolute error < ±18 kPa) in bench top mechanical tests and less than 21% variability within walking of 15-minutes duration. The results indicate the importance of mechanical coupling between embedded shear sensors and insole materials. It also highlights the importance of using an appropriate calibration method to ensure accurate shear stress measurement. The novel shear stress measurement system presented in this paper, demonstrates a viable method to measure accurate and repeatable in-shoe shear stress using the calibration procedure described. The validation and calibration methods outlined in this paper could be utilised as a standardised approach for the research community to develop and validate similar measurement technologies.
Citation: Haron AH, Li L, Shuang J, Lin C, Dawes H, Mansoubi M, et al. (2024) In-shoe plantar shear stress sensor design, calibration and evaluation for the diabetic foot. PLoS ONE 19(9): e0309514. https://doi.org/10.1371/journal.pone.0309514
Editor: Andrea Tigrini, Polytechnic University of Marche: Universita Politecnica delle Marche, ITALY
Received: January 16, 2024; Accepted: August 14, 2024; Published: September 4, 2024
Copyright: © 2024 Haron et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are available from the Mendeley Data database (DOI 10.17632/pcggh2rzm3.1 ). Only raw anonymised data can be shared due to GDPR restrictions from HRC ethics committee.
Funding: This work was partially funded by Engineering and Physical Sciences Research Council (EPSRC) grant number EP/W00366X/1.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Diabetic foot ulceration (DFU) affects 15–25% of people with diabetes at some point in their lifetime [ 1 ] and has a high social and economic cost with countries like the UK spending approximately £1 billion annually [ 2 ]. Worldwide the prevalence of diabetes is rising, and it is predicted that 552 million people will have the condition by 2030 [ 3 ]. Measurement of plantar normal stress and plantar shear stress has shown the potential to predict DFU risk [ 4 , 5 ]. However, whilst commercial systems are available to measure normal plantar stress in-shoe there are no commercially available in-shoe plantar shear stress measurement systems. Shear stress has been directly measured during barefoot gait using mechanical sensor arrays coupled with resistive or capacitive sensors [ 6 – 8 ], utilising piezoelectric materials and their charge outputs [ 9 ] and through a variety of optical methods including polycarbonate arrays [ 6 ], optical bend loss [ 7 ] and laser interferometry of bi-refringent films [ 8 ]. Perry et al. [ 10 ] used an array-based device [ 11 ] to study bunching and stretching of adjacent plantar tissue and they found that tissue stretching from shear stress was the predominant mechanism. They report that peak shear stress and peak plantar pressure occur in the same place in 50% of cases, but actually occur at different times, which is contradictory to results reported by other researchers [ 12 ]. Contradictory results are typical from these studies using custom-built shear stress measurement devices due to the relatively low numbers of participants with diabetes tested in the trials, with typical sample sizes of ten. All these measurement methods are bespoke devices and only a handful of foot-to-floor shear stress measurement devices exist worldwide. Larger scale studies with matched control groups are required to provide firm conclusions on plantar surface shear stresses experienced by people with diabetes.
Shear stress measurement is further complicated as all diabetic patients are strongly advised to walk using footwear (and never barefoot), therefore, to understand the shear stresses induced on the plantar surface, in-shoe shear stress measurement must be taken. Although direct shear stress measurement is important in DFU risk management, future use of artificial intelligence methods [ 13 , 14 ] may enable risk management with current measurement technologies.
In-shoe plantar shear stress is difficult to measure and reported measurements vary widely, for example, measurements of shear stress on the 1st metatarsal head varied from 16 kPa [ 15 ] to 140 kPa [ 5 ] in healthy participants. Therefore, either there is widespread inter-participant variability and/or there are mechanisms which cause errors for in-shoe shear stress measurement. Measurement error has been widely reported for in-shoe normal stress systems with causation linked to sensor wear and calibration [ 16 , 17 ]. Specifically, calibrating with similar load ranges to those desired to be measured improved accuracy by up to 20 times [ 16 ] and accuracy was reduced when smaller areas of loading were applied [ 17 ]. It is likely that similar calibration issues will affect in-shoe shear stress sensor measurement accuracy. Researchers have made excellent progress in developing novel in-shoe plantar shear stress measurement systems; however, they have not yet fully considered the implications of calibration methods on measurement accuracy [ 4 , 5 ]. The choice of indenter area of loading, shape and location is also an important consideration for accurate and reliable sensor calibration; despite this, to the authors’ knowledge this has not been investigated and reported in the literature. A key principle in calibration is that the applied loading should be a good representation of the real-world scenario. In the context of plantar foot mechanics, and for example the metatarsal heads, there is variation in the magnitude of loading, area of loading, shape and potentially the location of the bone in relation to the sensor. This paper presents the design and evaluation of an in-shoe shear stress sensor and considers the impact of calibration on measurement accuracy.
This paper describes a sensor system design and conducts a performance investigation. Three investigations were conducted: calibration investigation, loading profile comparison and sensor validation. These investigations and how they relate to one another are shown in Fig 1 .
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0309514.g001
Sensor system design
Sensing principle..
Coulomb’s law of friction describes frictional force being proportional to reaction load. In the case of shear sensing insoles this means that there can be no shear stress (friction) without normal stress (reaction load) and that the magnitude of associated shear stress will always be less than that of normal stress. Like most other shear sensors in the literature [ 5 , 15 , 18 – 22 ] the shear sensor is embedded in a hyperelastic or viscoelastic, isotropic incompressible elastomer, as opposed to a discrete sensor placed on the insole or isolated from the main body of the insole material.
Fig 2A shows a cylindrical section of elastomer insole with cross-sectional area, A, containing a strain gauge orientated in the shear plane and a normal stress sensor with sensor readings in mV, S, and N, respectively. The material properties (stress-strain relationship) for the silicone are non-linear but can be approximated as three linear regions (low: ≤ ε 1 = 0.04 strain ; medium: ≤ ε 2 = 0.115 strain ; high: > ε 2 strain ); see Fig 2B . The strains for the three linear regions were determined from the stress-strain curve of the silicone under compressive loading at the target stresses of 14 kPa (low), 70 kPa (medium) and 140 kPa (high). Stress-strain relationships for normal compressive loading are given by Eq 1 , where C medium , and C high are negative intercepts in units of pascal ( C low = 0) and E is the gradient in Pascal.
[A] Cylindrical section of elastomer containing strain gauge and normal force sensor [B] Stress-strain curve of the elastomer under compression stress. Linear approximations for deformation were made for three regions of the curve (low, medium, and high stress magnitudes), sectioned by the compressive strains ε 1 and ε 2 , with corresponding gradient E used for calibration. [C] Cylindrical section deformed by normal force only. [D] Cylindrical section deformed by both normal and shear forces.
https://doi.org/10.1371/journal.pone.0309514.g002
Fig 2C shows the section being loaded with a normal force which creates a reduction in thickness but an increase in diameter described by Eq 2 (assuming constant volume) which gives sensor readings S N and N N , which are signal voltage measurements (mV) for the shear stress and normal stress respectively, described by Eqs 3 and 4 where k N and k S are constants (sensor gains) determined by experiment with units Pa/mV and strain/mV respectively (other equation parameters defined in Fig 2 with SI units).
Fig 2D shows applied loading from both normal and shear force giving a sensor reading S N + S and N N for the shear stress and normal stress respectively. The applied shear stress, σ S , can be determined from Eq 5 and Eq 1 (assuming an isotropic material) which requires measurements from the normal stress sensor, N N , to decouple the effect on the strain gauge from normal force (where i = low , medium or high ).
Shear stress sensor design.
The shear stress sensing system primarily consists of the strain gauge rosette, a normal stress sensor, and the flexion stiffener and load concentrator; here on in referred to as the ‘shear stress system sensor’ or ‘SSS sensor’. A 3-element strain measuring rosette (1-RY81-3/120, Hottinger Bruel & Kjaer UK Ltd, Royston, England) was chosen for the shear stress sensor ( Fig 3A ) arranged in rectangular 0°-45°-90° directions to allow for calculation of resultant shear in both the anterior-posterior (AP) and medial-lateral (ML) directions. The sensor was then embedded in silicone (Sil A50 Smooth- Sil Addition Cure silicone, Smooth-On Inc. Macungie, USA). To assemble the sensor, the first 2mm silicone base layer was poured into a custom 3D printed square mould with dimensions of 20 x 20 x 4 mm (width x length x height). After curing the surface was cleaned and the strain gauges were soldered to 2-core 2.8 mm 2 external diameter shielded wires (JY-1060, Pro-Power by Newark, Chicago, USA). The strain gauges were then placed on the surface of the silicone using a custom 3D printed jig with tabs and bolts to align the strain gauges in the correct angular position. A thin second layer of silicone (approximately 0.5 mm thick) was then poured and allowed to fully cure, the jig was then removed and a final layer of silicone was poured on top to give a total thickness of 4 mm. A 15 mm diameter, 0.8 mm thick phenolic sheet material flexion stiffener and load concentrator was placed at the center of the sensor assembly and the top layer of silicone was then allowed to cure. The full assembly of the sensor is shown in Fig 3B and 3C .
[A] Configuration of the strain rosette in the sensor with three strain gauges arranged at 0°– 45° - 90°, and the relationship between the local strain axes and the global applied shear direction axes (Medial-Lateral, ML, and Anterior-Posterior, AP). [B] Section view of the SSS sensor. [C] Top view of the SSS sensor and its dimensions. [D] Locations of SSS sensors in the sensing insole.
https://doi.org/10.1371/journal.pone.0309514.g003
As mentioned in the sensing principle, the shear stress is obtained from Eq 5 , however, for the SSS sensor to measure both AP and ML shear stress, orientation of the strain gauges needs to be considered. From the configuration shown in Fig 3A for stress measurements calculated from strain gauges A, B and C the shear stress is given by Eqs 6 and 7 .
Where θ AB and θ BC are the angles between the individual strain gauges in the rosette, which were at 45°.
Shear stress sensor number, placement, and integration.
Key DFU risk areas, accounting for at more than one-third of DFU cases are the calcaneus, first metatarsal head and hallux areas of the foot [ 23 – 26 ], so placement of the SSS sensors in the insole was in these three locations. To maximize accuracy of the measured sensing data, all sensors were anatomically matched to the participant. This was achieved through a ‘palpation and marking paper’ approach in which a healthcare professional identified the bony landmarks of the foot, marked these areas on the foot surface with skin-safe marker, and the participant stands on the paper to transfer the markings. These markings were then used to ensure SSS sensors were correctly located on the silicone insole, with the sensor x-axis aligned with the anterior posterior direction. The signal wires were laid out from the SSS sensor in the ML direction to reduce fatigue loading from flexion during gait. A 1–2 mm depth of silicone was then poured and cured before a further layer of silicone was poured and cured to make a total insole thickness of 5 mm to complete the insole, as shown in Fig 3D . Three normal stress sensors (A301 FlexiForce 0-44N, Tekscan Inc., Norwood, Massachusetts, USA) were then secured to the bottom of the insole with silicone glue (Permatex 80050 Clear RTV Silicone Adhesive Sealant, Permatex, Illinois Tool Works Inc., Solon, Ohio, USA) with their center coincident with the SSS sensors.
Data acquisition system (DAQ) and signal processing.
A Teensy 4.1 32-bit microcontroller (PJRC, Portland, Oregon, USA), ARM Cortex-M7 processor, with clock speed of 600 MHz and integrated SD storage card, was used to collect and store the voltage readings from the SSS sensors ( Fig 4B ). Flexiforce normal stress sensors were connected via a 10 kOhm circuit divider to analog inputs, whilst shear sensing strain gauges were amplified using a 24-bit high-precision analog-to-digital amplifier (HX711 ADC, HALJIA, Zhongai, China) then routed to digital inputs of the microcontroller. All signals were collected at a sampling rate of 80 Hz. Data was logged to the 16GB SD card and streamed via an ESP8266 UART WiFi adapter (Espressif Systems, Shanghai, China) to allow for continuous monitoring. Power was supplied to all components via 3V and 5V power rails from the microcontroller, sourced from an external 3.7V 3500mAh Lithium Polymer battery (LP104567, EEMB, Moscow, Russian Federation) that was regulated through a linear regulator (LDO, B08HQQ32M2, DollaTek, Hong Kong, China). For both left and right foot measurements, two identical systems were used to collect the measurements, and placed on a custom, adjustable neoprene fitness belt (Frienda, China), during walking trials ( Fig 4 ).
[A] Participant walking on a treadmill with the sensor insole system. The data acquisition system (DAQ) was attached to a belt, and each insole (left and right foot) has a separate but identical DAQ input. [B] Block diagram of the DAQ system, collecting data at 80 Hz.
https://doi.org/10.1371/journal.pone.0309514.g004
A custom MATLAB (The Mathworks Inc., Natick Massachusetts, USA) script was used to parse and analyse the data collected. The data was minimally pre-processed before finalized into calibrated stress measurements. This pre-processing stage included removing only obvious outliers (which accounted for up to 0.05% of the measurement data if present). This was made using the filloutlier function with the ‘quartile’ outlier detection option: ‘quartiles’ outliers which were elements more than 1.5 interquartile ranges above the upper quartile (75 percent) or below the lower quartile (25 percent)) and correcting DC offsets. Data from each foot were analyzed separately.
Calibration investigation: Bench top mechanical testing
Experimental setup and test method..
To investigate the effect of calibration on the sensor’s performance, both shear and normal force were applied to the SSS sensor insole (summarised in Fig 1A ). A uniaxial mechanical testing machine (Instron 5982K2680 100kN 350°C, 500N load cell, Instron ® Norwood, Massachusetts, USA) applied and measured shear force using a bespoke shear stress rig through an indenter of area, A, shown in Fig 5A . A normal reaction force was applied through a screw thread to the indenter to facilitate frictional shear stress application. Measurement of normal reaction force was through a load cell and ADC (ADN1903027, 196.2 N Weight Sensor Load Cell, Haljia, China) capturing data at 80Hz using an Arduino (Arduino Mega 2560 Rev3, Arduino, Somerville, MA, USA). For pure normal stress loading calibration, the insole was placed flat on a plate in the uniaxial testing machine fitted with a large compression platen on the bottom and an indenter with a specific area, A, applying compression force from the top, shown in Fig 5B .
[A] Custom shear stress rig made of rigid 10 mm acrylic sheet plates which applied the force of the mechanical testing machine as a shear force onto the insole. The shear stress was calculated using the applied force and area of the custom indenter. The indenter’s compressive stiffness was 30.1 MPa, ~12 times stiffer than the silicone sensor of 2.5 MPa. [B] Custom normal stress calibration setup where the insole was placed on a compression platen.
https://doi.org/10.1371/journal.pone.0309514.g005
Sensor loading area investigation.
To evaluate the effect of indenter area, A, five flat ended cylindrical indenters with diameters of, 10, 15, 20, 25, 30 mm were used to load the SSS sensor at its center. While studies have shown that there is a difference between various indenter shape loading profiles and the corresponding mechanical responses of the material [ 27 , 28 ], we determined that the normal stress distribution that was measured at the surface of the SSS sensor was similar for both flat and rounded indenter profiles. The only notable difference was the size of the normal stress distribution, as a flat indenter covered a larger area than the rounded indenter of the same diameter. Thus, choosing a flat indenter of a smaller size gave the same loading results as a larger rounded indenter.
The tests applied a cyclic shear force with a 1 Hz triangular waveform pattern ranging from 0 to 50 N in combination with a constant normal stress of 140 kPa through all the indenters. SSS Sensor output signal, S N + S , in mV was measured for each of the loading areas.
Sensor loading location investigation.
Ideally a sensor would be co-located with the anatomical part applying the load, however, this may not always be practically possible so an understanding of the relationship between the location of the SSS sensor, the location of the applied loading and the accuracy of measurement is required. To investigate the effect of loading location, twelve loading locations were chosen, six in the anterior direction and six in the lateral direction both measuring 0, 10, 15, 20, 30, 40 mm from the center of the shear stress sensor. Loads were applied in both the medial or posterior direction respectively. Cyclic loading was applied to the SSS sensor insole of the same characteristic as the area of loading investigation (see ‘Sensing loading area investigation’ section). SSS Sensor output signal, S N + S , in mV was measured for each of the loading locations.
Loading profile comparison: Human plantar loading specific sensor calibration
Comparison of normal stress profiles..
Shear loading application area and location affect strain measurements, so it is important to consider plantar stress loading from the human foot. During walking plantar stress is dependent on many factors including foot size and anatomy, weight, morbidity and walking patterns, all of which are different between participants. From the sensor calibration investigations in the results section, we can see that (i) loading location and (ii) loading area may affect the output of the SSS sensor so these must be considered during calibration.
- Loading location variation can be removed by placing the SSS sensors at personalised anatomical locations in the insole, which is the approach we have taken.
- Loading area variation can be controlled through calibration. This was determined through a comparison and matching of normal stress loading profiles of the specific participant’s foot anatomy with bench top mechanical test experiments involving various loading area sizes (flat cylindrical indenters).
To capture the plantar normal stress loading profiles of our participants, in the SSS sensor locations of the calcaneus, first metatarsal head and the hallux, we conducted measurements in-shoe during a two-minute treadmill walk using an F-scan insole (Tekscan Inc., Boston, USA) coupled with a non-instrumented insole of the same material properties and thickness as our designed insole. Then the test rig ( Fig 4B ) was used with 15, 20, 30 and 40 mm diameter indenter sizes to load the silicone insole from 0 to 250 N (to simulate a normal stress range up to 1400 kPa, which is comparable to the 1000–1900 kPa normal plantar stresses during gait reported in the literature [ 29 , 30 ].
Measurements of plantar normal stress distribution were captured with the same F-scan and insole used with the participants. To simulate the different foot structures, we adjusted the diameter of cylindrical indenters (15, 20, 30 and 40 mm), which were based on the ranges of average anatomical dimensions of the hallux, metatarsal head, and calcaneus bones [ 31 – 36 ], see results and discussion ‘Human plantar loading consideration for sensor calibration’ section. An illustrated summary of this investigation can also be found in Fig 1B .
Statistical analysis as a method for calibration indenter choice.
Comparisons were made between the participant’s mean normal stress profiles with the bench top test rig results (gait data averaged over 20 gait cycles from three different sensing locations hallux, first metatarsal head, and calcaneus, bench top test rig results for 15, 20, 30 and 40 mm indenter diameters). Magnitudes of both results were scaled to have a maximum unity magnitude to enable comparison. The normal stress profiles (normal stress vs displacement across anatomical location) were collected along a 2D cross section of 40 mm in length across the foot-width of loaded area (see results and discussion ‘Human plantar loading consideration for sensor calibration’ section). Calibration indenter diameters for the hallux, first metatarsal head and calcaneus locations were chosen based on either the highest R 2 value from a multiple linear regression between the gait measures and the test rig measures or the maximum measurement sensitivity area of the SSS sensor (see results and discussion ‘Sensor calibration’ section).
Sensor validation: Bench top mechanical testing
The following section describes the sensor validation, as summarised in Fig 1C . A 30 mm diameter indenter was used to calibrate the SSS sensor, as this was determined to be the maximum sensing area of the sensor (see results and discussion ‘Sensor calibration’ section). This was achieved through a series of mechanical tests detailed in Table 1 , with shear stresses applied in both ML and AP directions and conducted at 1Hz, to simulate average walking speed frequency.
https://doi.org/10.1371/journal.pone.0309514.t001
The shear stress magnitudes chosen for low, medium, and high levels were 10%, 50% and 100% of the 140 kPa maximum in-shoe plantar shear stress reported in the literature respectively [ 37 ]. This enabled calculation of the calibration parameters coefficients E low , E high , C medium and C high , according to Eq 1 .
To validate the calibrated SSS sensor, a shear stress of 70 kPa with a normal stress of 125 kPa was applied in both the ML and AP direction at 0.8 Hz. Additionally, a shear stress was also applied in the 45° direction (14 kPa shear stress, 28 kPa normal stress at 1Hz).
Two measurements of error were made. The first was an overall mean absolute error (MAE), which is the mean of the difference between the measurement from the test rig and the calibrated SSS sensor measurement (in kPa). The second was peak error, measured as the percentage error at peak loads between the applied measurement from the test rig and the calibrated SSS sensor measurement. Peak values of measured shear stress were taken from 10 cycles and a standard deviation was calculated. Repeatability was calculated from the SSS sensor measurements as the standard deviation of the peak plantar stresses divided by the mean of the peak plantar stress, presented as percentage (e.g. a mean peak measurement of 100 kPa and a standard deviation of those peak measurements at ± 10 kPa, would result in (10/100) x 100% = 10% deviation from the peak value, and thus 90% repeatability).
Sensor validation: Gait lab treadmill walking
To further validate the sensors, a gait laboratory treadmill walking test was performed on a single anthropometrically matched healthy participant and a single participant with diabetes (both male and 45 years old, weighing 88 kg and 75 kg, height of 1.75 and 1.66 m, EU shoe size 44 and 42, weight per insole area 32 kPa and 35 kPa, walking speed 0.92 ms -1 and 0.95 ms -1 for the healthy participant and participant with diabetes respectively). The study received approval from the NHS Health Research Authority and Health and Care Research Wales (HCRW) Ethics Committee (REC reference: 22/NW/0216), and all participants provided written consent. Trial Registration number: NCT05865353. Participants were recruited between 1 st November 2022 till 30 th May 2023. Data collection was conducted in two parts (1) baseline visit and (2) main data collection, Table 2 .
https://doi.org/10.1371/journal.pone.0309514.t002
Baseline visit.
Anthropometric data was collected, and anatomical landmarks determined using the ‘palpation and marking paper’ method described in the ‘Shear stress sensor number, placement and integration’ section. The participants conducted a 2 minute treadmill walk while wearing a pair of silicone insoles, made from the same materials and dimensions as the sensor insole but without active sensors, and a pair of F-Scan pressure sensing insoles, in a prophylactic shoe (Sponarind 97308, Finn Comfort Inc. Hassfurt, Bavaria, Germany), designed with shock-absorbing properties and a larger volume, ideal for people with diabetes. Normal stress data was collected using the F-scan insoles, at a self-selected gait speed to determine normal plantar stress profiles (results of which were used for the comparison of normal stress profiles, in ‘Human plantar loading specific sensor calibration’ section). Table 2 shows the participant data collected during the baseline visit.
Main data collection.
The participants returned for the main data collection where they were asked to wear the sensing insole in the specialist diabetic shoe. They then walked twice on a split belt treadmill with integrated force plates (M-Gait, Motek Medical BV, Amsterdam, Netherlands) for 15 minutes at their self-selected speed (see Table 2 ).
Data analysis: Shear stress gait measures and repeatability.
Mean and standard deviation of peak shear stress and peak normal stress measurements were extracted from 20 gait cycles measured by the sensing insole. Measurement repeatability was determined and comparisons, between the two walking periods within each individual walking session (start, middle, and end). We collected statistical data for both plantar shear stress and normal stress measurements to perform inter-participant comparisons. These included statistics for Plantar Stresses (Normal, AP Shear, and ML Shear) across all three sensor areas, encompassing mean values, standard deviations, peak stresses, and variability (or percentage difference) of measurements within the 15-minute treadmill walk (intra-walk) and between two treadmill walks (inter-walk).
Results and discussion
Sensor calibration.
Shear stress measurement accuracy is affected by the calibration method. Specifically, the shear stress sensor measured output signal decreases exponentially with both increasing loading application area, and increasing loading distance away from sensor center, see Fig 6 . The results in Fig 6A show that the measured output decreases by ~80% from 1.5 mV to 0.3 mV, for a calibration loading application area of 10 mm diameter to 30 mm diameter respectively. This means that if the sensor was calibrated for the smaller 10 mm area and a larger 30 mm diameter load was applied, the measurements would be underestimated by 80%. Likewise, calibrating for a larger area, and applying load for a small area will greatly overestimate the measurements. Increasing the loading application area increases the area over which the force is distributed over the sensor, thus more of the loading is applied away from the center of the shear stress sensor. From the results shown in Fig 6B and 6C the location of loading application also reduces sensor sensitivity. All this means that the shear stress sensor will only be able to measure accurately if the calibration loading area matches the desired measurement loading application area (or are reasonable similar areas).
Mean peak signal of shear stress (SSS sensor) total output (mV) from 10 cyclic triangular loading. [A]—Effect of area of loading on SSS sensor measured outputs. [B, C]- Effect of location of loading on SSS sensor output for medial and posterior respectively.
https://doi.org/10.1371/journal.pone.0309514.g006
Fig 6B and 6C show the influence of loading location on SSS sensor measurements for the same applied loading area (25 mm diameter indenter). As expected, the SSS sensor measurement for both Anterior-Posterior (AP) and Medial-Lateral (ML) shear loading decreased as the loading distance moved away from the sensor center. This is due to a decrease in deformation of the shear stress sensor as the loading is applied further away from the sensor center. However, it is important to note that there was still a measurable signal at these distances as they are not yet relatively far away from the sensor. This means that measured shear stress from an embedded sensor will not just be from the coincident anatomical location but also have a contribution from adjacent and other relatively close anatomies (e.g. first metatarsal head located sensor may be measuring shear stress contribution from the second metatarsal head). This is due to material coupling, which is that stress applied in one area of the material, in this case the silicone insole, will stress surrounding areas of the material. The implication is that the shear stress sensor will provide more accurate measurements if the loading application location is coincident with the centre of the sensor. This emphasizes the importance of the placement of these discrete sensors, which is why a participant specific sensing insole was manufactured, placing sensors at the exact anatomical location of the boney landmarks, where peak loading is expected.
Although this paper presents the shear stress sensor sensitivities to calibration loading area and calibration loading locations for this sensor it is likely that these observations are true for other embedded in-shoe shear stress sensors. Other researchers measured in-shoe peak shear stresses from gait varied from 9 kPa to 140 kPa and calibration loading area varied from 20 mm diameter area (314 mm 2 ) –10,000 mm 2 (up to half the insole, approximated from the experimental Fig 3 in the paper as there was insufficient detail to give conclusive information on the loading area used) [ 5 , 15 ]. It is likely that these variations in measurements are not due to inherent sensor inaccuracy or participant gait differences but likely to stem from calibration method differences. To the authors’ knowledge, calibration loading area has not been investigated in other published studies, but it is suggested that calibration should be considered for all future in-shoe shear stress measurements.
Human plantar loading consideration for sensor calibration
Fig 7 shows that calibration loading indenter diameters should be 20 mm and 40 mm for the hallux and both the first metatarsal head and the calcaneus respectively. However, due to limitations on sensor sensitivity beyond 30 mm from the center of the sensor a 30 mm indenter diameter was chosen for the first metatarsal head and calcaneus. These choices of calibration indenter diameters were determined from the comparison of the bench top testing normal stress profiles of different indenter diameters, with the participants’ measured normal stress profile during walking. The bench top test showed that all the indenters resulted in normally distributed normal stress profile curves ( Fig 7A ), increasing in curve width with increasing indenter diameters, reflecting a larger contact area of the applied force. An increasing curve width is also expected for the normal stress profiles of anatomical bones with increasing diameters (first metatarsal head ~15 mm, hallux ~20 mm, and calcaneus ~ 40 mm [ 31 – 36 , 38 ]). The participants’ measured normal stress for the hallux and the calcaneus regions of the foot had normal pressure distribution profiles that reflected their anatomical sizes, however, the presence of the second close metatarsal bone influenced the normal stress profile in the first metatarsal head area and widened the normal stress profile, more than what is expected from its anatomical diameter of ~15 mm ( Fig 7B ). The R 2 results of the multiple linear regression reflected this ( Fig 7C ), as the first metatarsal head correlates to the indenter size of 40 mm diameter. The R 2 value of the metatarsal head, however, is small at 0.41, indicating that there may be variability in the pressure distributions in that area, likely from gait variability within a participant’s walk or between participants. The hallux and calcaneus regions of the foot have a normal pressure distribution profile that reflects the loading of the anatomical bones clearly (R 2 ≥ 0.95) and can be matched with an indenter of a similar size to give a representative loading for calibration of 20 mm and 40 mm respectively. However, loading area results from Fig 6A show that sensor sensitivity converges for indenter areas greater than 25–30 mm diameter. Therefore, calibration indenter diameters were reduced to 30 mm for the first metatarsal head and calcaneus.
[A] Experimental normal pressure profiles: (i) Indenter experimental setup, (ii) Normal pressure profile curves width increases with increasing indenter diameter, (iii) F-scan pressure result that shows the cross section used to obtain these values used in ii. [B] Participant pressure profiles: (i) In-shoe gait lab experimental setup (ii) An example of participant’s pressure profile over 20 gait cycles, showing the three normal pressure profiles of the foot at the calcaneus, first met head and hallux, (iii) F-scan pressure result that shows the cross section used to obtain the values. The image also shows the peaks for these three regions (Calcaneus peak CP, Hallux Peak HP and the metatarsal peaks MHP1 and MHP2). [C] Graphical representation of the regression analysis’ coefficient of determination (or R-squared) results. Larger circles indicate a higher R-squared value, and red circles indicate the maximum R-squared in the sensor group. R-squared values are shown above the circles, and maximum is indicated as red font.
https://doi.org/10.1371/journal.pone.0309514.g007
The implications of this for the SSS sensor are that calibration indenter sizes should be between 10–30 mm dependent on expected shear stress application areas. This finding is likely to be true for other embedded in-shoe shear stress sensors in the literature. The limitation from this finding is that to obtain accurate shear stress measurements the user must know something about the shear stress loading profile which may be unknown. A possible way to mitigate for this may be to calibrate the sensor for a range of loading areas and to use a normal stress sensor to determine which indenter calibration area to use in post-processing.
Shear sensor calibration and bench top mechanical test validation
The SSS sensor was highly accurate and repeatable when compared against the bench top mechanical test as seen in Fig 8 . Results from Table 3 show that calibration error was insignificant with the mean absolute error (MAE) over the entire cycle in calibration < 0.00007 kPa for all magnitudes of loading, and errors at peak loading were < 5.8%.
[A] Sensor calibration for both anterior-posterior (AP) and medial-lateral (ML) directions at a ‘medium’ level of posterior and medial shear loading of 1 Hz cyclic loading of up to 70 kPa shear stress, at a constant normal stress of 140 kPa. [B] Sensor validation test result at medium level of shear cyclic loading (up to 70 kPa), at a different loading frequency (~0.85 Hz) and different constant normal stress (125kPa). All results for the different configurations of loading are shown in Table 3 .
https://doi.org/10.1371/journal.pone.0309514.g008
https://doi.org/10.1371/journal.pone.0309514.t003
The errors in the validation of the sensors at loading conditions different from the calibration were higher, but still showed a high accuracy for the sensors. The sensor was most accurate for low–medium shear stress magnitudes with up to <1.8 kPa for MAE, and < 8.7% for error at peak loading (see example of medium magnitude measurements in Fig 8 ). Followed by the measurements at a resultant loading angle of 45° clockwise from the anterior direction (MAE <1.4 kPa; <11.5% peak error). These small errors could be attributed to errors in the validation setup, as an error of ± 5° would correspond to a peak shear stress error of up to 4.6%. The SSS sensor also showed good repeatability for all loading conditions (>97% repeatability in calibration and >96% repeatability in validation).
The highest errors in validation were at high shear stress magnitudes, over the expected plantar shear stress from gait, these were MAE <17.3 kPa and peak error <22.4%. This was likely due to the mechanical coupling of the high normal stress, pushing the total material deformation higher up the hyperelastic stress-strain curve of the sensor material ( Fig 2D ). At this region of the stress-strain curve, very small strains relate to high changes in stress making the SSS sensor more prone to measurement errors. However, the maximum errors translate to an error of ± 31.3 kPa, which is within the standard deviation of most plantar stress measurements from the literature of ± 50 kPa for shear stress [ 1 – 5 , 15 ].
Treadmill walking validation
For treadmill walking the SSS sensors measured the magnitude of shear stresses between 66.5 kPa—152.6 kPa in the AP direction, and 28.4 kPa– 128 kPa in the ML direction, full results are shown in Table 4 . As expected, the ML shear range was lower than the AP shear range, as loading was expected to be predominantly in the AP direction. Loads were cyclic going from zero to peak value with the same frequency as gait which were at speeds of 0.92 and 0.95ms -1 for the healthy participant and participant with diabetes respectively. The only notable differences were in the direction of some of the peak plantar shear stresses.
https://doi.org/10.1371/journal.pone.0309514.t004
No significant differences between both participants peak plantar stress values were observed (t-test of mean peak plantar stresses PPS, p>0.36, p>0.58 and p>0.57). This was expected, as both participants had a similar walking speed (0.92–0.95 ms -1 , and weight per insole area 32.4–35 kPa). However, this study aimed to demonstrate the feasibility, accuracy, and repeatability of the SSS system so no conclusions should be drawn on plantar stress for general people with diabetes and healthy populations for this study.
The shear measurements of the SSS sensor was highly repeatable when comparing data recorded for both within the 15-minute treadmill walk (intra-walk), and between the two 15-minute walks (inter-walk). The mean and standard deviation of the percentage difference of peak plantar stresses were ≤ 8% ± 6% for both investigations. Intra-walk differences were lower than inter-walk–with the highest percentage difference of 21% measured by the SSS sensor for the ML Shear (Hallux, Left foot, participant with diabetes). Other measurements from the shear stress sensors were < 15% difference. For inter-walk, the highest PPS percentage difference was measured by the commercial Flexiforce sensor of 47% difference in normal stress (Hallux, left foot, participant with diabetes), followed by 37% for the AP shear of the SSS sensor (Hallux, right, healthy) and 33% for the ML shear of the SSS sensor (Calcaneus, right, participant with diabetes).
Calibration and material coupling for shear stress sensors
To the author’s knowledge, this study is the first to address in-shoe shear sensing material coupling and unexplored complexities in calibration for shear sensing. The results illustrate that due to sensor and material coupling with adjacent structures the area which contributes to the measured shear can be larger than the area of the sensor. This has important implications for shear sensor calibration, firstly in terms of the location of the sensor and the anatomical region that is to be measured, and secondly in terms of the indenter area used for calibration. These results have significance for all researchers developing systems to measure in-shoe plantar shear stress as these factors will affect the magnitude of shear sensed. Furthermore, these results may partially explain the variation in magnitudes of shear measured at the same anatomical locations by different researchers. A suggested approach for shear sensor calibration is shown below (for detail see methods ‘Human plantar loading specific sensor calibration’ section):
- Determine the sensing area : Material coupling between the shear sensor and adjacent regions can result in the area sensed being greater than then actual area of the sensor.
- Determine the distribution of plantar loading : Normal stress distribution will be indicative of shear stress distribution, whilst foot anatomy, for example the hallux, will determine the loading area.
- Decision for calibration indenter area : Informed by both the sensing area and the distribution and magnitude of plantar loading.
Developed shear stress system sensor
Sensor performance..
A novel Shear Stress System (SSS) sensor composed of a strain gauge rosette, normal pressure sensor and stiffener to concentrate loading at the desired sensor location and mitigate against material coupling was developed and evaluated. Sensor locations were anatomically matched and measured the plantar loading profiles to inform calibration of each sensor at a specific location. This study conducted a thorough experimental validation of the shear sensor through mechanical bench top testing and with human participant treadmill walking. Shear sensing results demonstrated high repeatability (>97%) and high accuracy in the expected measurement range for plantar shear stress (mean absolute errors < ±2 kPa) with error increasing for very high shear stresses (mean absolute errors < ±17 kPa) compared to bench top mechanical tests and repeatability for treadmill walking of 15-minutes duration with less than 21% variability within walking, and less than 37% variability between walks (which was lower than the commercial normal pressure sensors of 47% used in this study).
Limitations.
A rosette strain gauge was chosen for determining unknown principal directions, however it restricted complete strain separation in the AP and ML directions. For exclusive separation, a 0°–90° strain gauge in the ML and AP axes could be adopted. The manual assembly of the sensors and alignment of the sensor in relation to the AP and ML directions affect shear measurement. This has been controlled through careful manufacture, but some small errors will remain. The chosen alignment of the strain gauge rosette in the ML direction was to reduce the fatigue on the soldered joints, this resulted in a decreased sensitivity in the AP direction due to the 45° off-alignment of the gauges with this axis.
Relative stiffness of the silicone and the strain gauge rosette will affect strain transfer between the two materials. Material properties of the silicone is highly important for measurement accuracy, sensitivity, and range, and warrants further investigation.
Future work.
A three-part linear fitting procedure was adopted to calibrate the SSS sensor accommodating the hyperelastic material properties, in the future consideration of alternative fits to capture viscoelastic effects could be made. Despite observing minimal shear sensor temperature response, variability between 20–30°C, literature indicates foot temperatures may be as high as 35° in people with diabetes [ 39 , 40 ], this should be considered in the future. In this proof-of-concept study, the size of calibration area was based on average pressure profiles, a suitable assumption with little participant variation. However, future larger studies may require participant-specific calibration to address varying loading profiles, particularly due to gait variability.
- View Article
- PubMed/NCBI
- Google Scholar
- 22. Amemiya A, Noguchi H, Oe M, Sanada H, Mori T. Establishment of a measurement method for in-shoe pressure and shear stress in specific regions for diabetic ulcer prevention. 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE; 2016.
IEEE Account
- Change Username/Password
- Update Address
Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Diabetic Attacks and Emergencies
Understanding blood sugar, diabetic ketoacidosis, hypoglycemia, hyperglycemia, increased risk of infections, diabetic coma, preeclampsia, heart attack or stroke.
A diabetic attack occurs when your blood sugar spikes too high or drops too low. This can cause a medical emergency.
A number of different conditions and factors can lead to a diabetic emergency, including ketoacidosis , hyperglycemia , and hypoglycemia . Each of these needs to be handled in a specific way to reduce the risk of long-term consequences.
This article explains types of emergencies that can result from diabetic attacks, their symptoms, and treatment options. It also covers possible complications and how to prevent attacks and problems.
Diabetes is a chronic condition where the blood sugar level is too high. Insulin, a hormone produced by the pancreas , removes sugar from the blood and moves it into cells for the body to use. In people with type 1 diabetes , their pancreas doesn’t make any insulin; in those with type 2 diabetes , it doesn’t make enough.
Having high blood sugar for a long period of time puts people with diabetes at risk for other health problems, such as kidney disease, heart disease , stroke , and nerve damage.
A diabetic emergency happens when blood sugar is too high or too low for too long. This is a life-threatening condition that requires immediate medical treatment. There are a few types of diabetic emergencies, and some conditions may increase the risk of a diabetic emergency.
Diabetic ketoacidosis (DKA) occurs when the body begins burning fat, instead of sugar, for fuel. This happens when there isn’t enough insulin to deliver sugar to cells for energy.
To make up for this, the liver begins breaking down fat too quickly for the body to process. This can lead to a buildup of ketones (a type of acid) in the blood, which can become poisonous.
Symptoms of DKA can include:
- Rapid breathing
- Flushed face
- Nausea, vomiting, or abdominal pain
- Decreased alertness
- Frequent urination or thirst that lasts for a day or more
- Dry skin or mouth
- Muscle stiffness or aches
- Dehydration
- Fruity breath
DKA is most common in individuals with type 1 diabetes. It can sometimes be the first sign of type 1 in those who are not diagnosed. Causes of DKA in type 1 diabetes include infection, injury, serious illness, missed insulin doses, or stress due to surgery.
DKA is less common in people with type 2 diabetes. If it occurs, it is typically less severe. Causes of DKA in type 2 diabetes include uncontrolled high blood sugar for a long period of time, missing medicine doses, or a severe illness or infection. It can also be a side effect of some medications for diabetes, such as sodium-glucose transport protein 2 (SGLT2) inhibitors.
When you eat too much sugar, the excess is stored in the muscles and liver. When blood sugar decreases, the liver releases what it has stored, raising the amount of sugar in the blood. For some, especially those with diabetes, their blood sugar doesn’t go up enough and is below 70 mg/dL, causing hypoglycemia, or low blood sugar.
Possible symptoms of hypoglycemia include:
- Fast breathing
- Sweating or chills
- Fast heartbeat
- Lightheadedness or dizziness
- Irritability
- Color draining from the skin
- Blurred vision
- Tingling or numbness in the lips, tongue, or cheeks
- Coordination problems
Hypoglycemia can happen to anyone, but for people with diabetes, hypoglycemia can occur as a side effect of the medicine they’re taking. Eating foods high in carbohydrates usually helps raise your blood sugar to normal levels.
If hypoglycemia happens too often, they need to consult with their healthcare provider to see if they need to change their treatment plan.
Hyperglycemia is blood glucose greater than 125 mg/dL while fasting, which is defined as not eating for at least eight hours.
It can occur in people with diabetes if they’re eating too many carbohydrates, taking their medicine incorrectly, or if their medication is not as effective as it should be.
Stress and the dawn phenomenon (a surge of hormones that leads to high blood sugar in the morning), could also lead to hyperglycemia.
Symptoms of hyperglycemia can include:
- Increased urination or thirst
- Slow-healing cuts and sores
Hyperglycemic hyperosmolar syndrome (HHS) can occur if you have a high blood sugar level for a long time. Signs of HHS can include:
- Blood sugar over 600 mg/dL
- Extreme thirst or dry mouth
- Confusion, hallucinations, drowsiness, or passing out
- Fever over 100.4 degrees F
- Weakness or paralysis on one side of the body
- Frequent urination
HHS usually develops in people who do not have their type 2 diabetes under control and who have an infection, stopped taking their medications, have a heart attack or stroke, or take medicine that can cause this condition, such as steroids and diuretics.
High blood sugar can negatively affect the immune system. It can lower the ability of white blood cells to come to the site of an infection and kill what is causing the infection. Nerve damage and difficulty breaking down and storing fats can contribute to an increased risk of infection.
People with type 1 or type 2 diabetes are vulnerable to infections that can become life-threatening, including:
- Fungal infections, such as jock itch, athlete’s foot, ringworm , and vaginitis
- Urinary tract infections
- Bacterial infections of the skin and soft tissue that won’t heal
Signs of infection can include fever, chills, sore throat or mouth sores, redness or swelling, or pain with urination.
A diabetic coma , where a person passes out due to extremely low or high blood sugar, is an emergency that requires immediate medical attention. Extreme hypoglycemia or hyperglycemia can cause a diabetic coma, so symptoms of these two conditions could be warning signs of this diabetic emergency.
Other circumstances can also increase the risk of diabetic coma, such as:
- Surgery or other bodily trauma
- Illness or infection
- Drinking alcohol
- Skipping insulin doses
- Poor diabetes management
Diabetic ketoacidosis and hypoglycemia are more likely to cause a diabetic coma in those with type 1 diabetes, while HHS places people with type 2 diabetes more at risk of this condition.
When to Call Your Healthcare Provider
You should call your healthcare provider or 911 if you have diabetes and the following:
- Your blood sugar is 300 mg/dL or higher two times in a row for an unknown reason.
- You have low blood sugar that has not come up after three treatments.
Preeclampsia is pregnancy-induced high blood pressure ( hypertension ) and liver or kidney damage. It often occurs after the 20 th week of pregnancy. The risk of preeclampsia is two to four times higher among people with type 1 or type 2 diabetes. Gestational diabetes , a type of diabetes that occurs during pregnancy, also increases your risk of developing preeclampsia.
The exact cause of preeclampsia is unknown. It is estimated to occur in about 3% to 7% of all pregnancies.
Women with preeclampsia often do not feel sick, but symptoms in the early stages could include:
- Swelling of the hands and face or eyes
- Sudden weight gain over one to two days or more than two pounds a week
- Headache that does not go away or becomes worse
- Trouble breathing
- Belly pain on the right side, below the ribs
- Not urinating very often
- Nausea and vomiting
- Vision changes, such as temporary blindness, seeing flashing lights or spots, sensitivity to light, and blurry vision
- Feeling lightheaded or faint
Even when diabetes is controlled, high blood sugar can still damage the blood vessels and nerves of the heart over the years. The longer you have diabetes, the higher the chances that you will develop heart disease . This increases the risk of heart attack or stroke.
Signs of a heart attack can include:
- Pain or pressure in your chest that lasts longer than a few minutes or goes away and returns
- Pain or discomfort in one or both arms, or the shoulders, back, neck, or jaw
- Shortness of breath
- Sweating or lightheadedness
- Feeling extreme fatigue
- Indigestion or nausea
Women are more likely to experience nausea or vomiting, back or jaw pain, and shortness of breath as heart attack symptoms.
Signs of a stroke are:
- Sudden numbness or weakness on one side of the body
- Trouble seeing or walking
- Sudden severe headaches with no known cause
- Confusion, difficulty speaking or understanding speech
If you experience any of these symptoms, call 911 immediately.
To avoid a diabetic emergency, you must manage your diabetes as well as possible. Check your blood sugar often, and get into the habit of recognizing the early signs that levels are rising or dropping toward a dangerous range.
Other tips to prevent a diabetic emergency include:
- Eat regularly and avoid foods that are processed or have added sugar
- Stay active and exercise regularly
- Take medications as prescribed
It’s also a good idea to carry snacks that you can eat to quickly get sugar into your blood to treat hypoglycemia. These might include raisins, candy, or glucose tablets.
For hyperglycemia, exercise will lower your blood sugar, but if your blood sugar is above 240 mg/dL, you need to check your urine for ketones. Exercising with a high ketone level will raise your blood sugar even higher.
If you are pregnant, your healthcare provider may recommend that you take daily low-dose aspirin to help prevent preeclampsia and its related complications. It is started between 12 to 28 weeks of pregnancy, but it is best to start before 16 weeks of pregnancy.
Diabetic attacks can be caused by hypoglycemia (low blood sugar) and hyperglycemia (high blood sugar), which can cause medical emergencies. Too little insulin can also cause an emergency condition known as diabetic ketoacidosis. During pregnancy, high blood pressure can also put you at risk for a diabetic attack.
Uncontrolled diabetes results in more than a diabetic attack, though. It also increases susceptibility to infections and puts you at risk for suffering from a diabetic coma.
There are steps you can take to reduce your risk and maintain a stable sugar level.
A Word From Verywell
Managing diabetes and the possibility of diabetic emergencies can feel overwhelming, but these emergencies are largely preventable by keeping your condition under control.
Eating healthy, taking medicines as prescribed, exercising regularly, and recognizing the early signs of rising or falling blood sugar levels can help you keep these emergencies at bay and become prepared in the event that they do occur.
National Institutes of Health. What is diabetes ?
American Diabetes Association. Hypoglycemia (low blood sugar) .
MedlinePlus. Diabetic ketoacidosis .
National Institute of Diabetes and Digestive and Kidney Diseases. Hypoglycemia .
American Diabetes Association. Hyperglycemia (high blood sugar) .
MedlinePlus. Diabetic hyperglycemic hyperosmolar syndrome .
Stoner GD. Hyperosmolar hyperglycemic state . Am Fam Physician ; 96(11):729-736.
Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study . Diabetes Care . 2018;41(3):513-521. doi:10.2337/dc17-2131
Centers for Disease Control and Prevention. Know the signs and symptoms of an infection .
Cleveland Clinic. Diabetic coma .
Weissgerber TL, Mudd LM. Preeclampsia and diabetes . Curr Diab Rep . 2015;15(3):9. doi:10.1007/s11892-015-0579-4
MedlinePlus. Preeclampsia .
National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes, heart disease, and stroke .
American Heart Association. Heart attack symptoms in women .
Centers for Disease Control and Prevention. Stroke signs and symptoms .
American Diabetes Association. Eating well .
U.S. Preventive Services Task Force. Aspirin use to prevent preeclampsia and related morbidity and mortality: U.S. Preventive Services Task Force recommendation statement .
By Carisa Brewster Brewster is a freelance journalist with over 20 years of writing experience specializing in science and healthcare content.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Ayurveda Integr Med
- v.9(4); Oct-Dec 2018
Integrative approach for diabetic foot management– a case report
Non-healing diabetic foot has always demanded more attention from the surgeon due to the distinct problem it possesses. There is a constant need for the evolvement in the management and this case is one such attempt. A 62-year-old male patient with a history of diabetes mellitus since 10 years presented with a non-healing foot ulcer since 1 month. He was managed with Ayurveda internal and external interventions for dusta vrana combined with contemporary methods like vacuum assisted wound suction and skin grafting. The patient who presented with non-healing diabetic foot was found to have better wound healing with combined intervention. The current approach indicates the better outcome with multi-dimensional approach towards diabetic foot.
1. Introduction
Diabetic foot is usually caused by a combination of three factors-ischaemia secondary to atheroma, peripheral neuropathy which leads to trophic skin changes and immunosuppression caused by excess of sugar in the tissues which predisposes to infection [1] .
Approximately 8% of diabetic patients have a foot ulcer and 1.8% has an amputation done [2] . With diabetes being a fast-growing disease, the importance of a better care has far more importance than before.
Diabetic foot management in the contemporary science include drainage of pus, debridement of dead tissue with local amputation of necrotic digits and antibiotics [1] . Similarly, in Ayurveda Shasti upakramas (60 interventions) have been mentioned to treat different types of wound based on their presenting symptoms [3] .
Both the sciences have described the management in depth, but there are limitations on either side. However, when used together, better outcome was seen in terms of the wound management.
In the present case, patient was managed with both Ayurveda and Allopathy conveniently along with other techniques which latest bio-medical engineering can provide.
2. Patient Information
A 62-year-old male patient approached the surgical unit of our hospital on 23/01/16 with a foul smelling, non-healing, necrotizing ulcer over the dorsal aspect of the right foot since 1 month. He was a known case of diabetes since 10 years which was not under control in spite of using tablet Glyciphage 500 mg orally all these years.
Patient was apparently normal 1 month before. A month back he noticed a spontaneous small opening without any known external injury-over the dorsal aspect of the right foot with mild discharge which gradually increased leading to a wide-open wound. According to the patient, the discharge was foul smelling and continuous. Wound was painless and spreading in nature. History of intermittent fever, nausea, vomiting, cough, difficulty in breathing and sleeplessness was present. There was no history of injury before the onset of the ulcer. Patient took treatment from a local physician with minimal relief. Since the wound showed no signs of improvement he was referred to a vascular surgeon where he was advised to undergo above ankle amputation. Patient was unwilling to undergo amputation and hence he sought an alternative approach.
3. Clinical findings
Local examination: Location- Dorsum of the right foot, infero-lateral aspect, Size- 5*9 cm, Floor-sloughed, tendons exposed, Edges-sloped and well defined, Margins – poorly defined at the distal ends, thick and fibrosed proximally, Discharge-purulent, Smell-foul, Blackish discoloration of the 4th toe, Surroundings-edematous with rise in local temperature, Peripheral pulsation- Dorsalis pedis, posterior tibial and popliteal artery pulsation well appreciated, Touch on bleed-absent.
General examination: Appearance- Distressed, Body built & strength- Moderate, Orientation-well oriented to time, place and person. Pallor (conjunctiva)- present, Icterus- Absent, Edema (local)- Mild swelling over the right leg lower 1/3rd, Lymph nodes- 2–3 right inguinal lymph nodes discrete, palpable and non-tender. Gait-limping gait.
Systemic examination: Respiratory system- Cough-present- increases at night, Phlegm-absent, Rhonchi-present.
On 23rd of January 2016, when the patient first visited the hospital, his hemoglobin was 9.8 gm %, Total count was 15,400 and ESR 120, Fasting blood sugar 229 mg/dl and post prandial blood sugar was 342 mg/dl.
Arterial doppler revealed – moderate atherosclerotic changes with no obvious obstruction.
Chest X ray- normal study
4. Therapeutic focus and assessment
For the above clinical presentation, procedures such as chedana (excision) [4] , bhedana (incision) [4] , vasti (medicated enema) [5] , parisheka (wound wash) [6] are indicated in Ayurveda. Initially the patient was started with Adhoshaka abhyanga (downward massage of the lower limbs with medicated oils), naadi sweda (steam) and panchavalkala kwatha avagaha (immersing the foot in medicated decoction) externally; Amruthotharaam kashayam and Gandhaka rasayana internally. The external treatments were done to enhance the blood circulation to the affected part, vaso dilatation with local steam therapy, wound cleansing, auto debridement and to initiate the wound healing ( Table 1 ). Patient's regular medications for diabetes were allowed to continue. However, as the patient was continuously febrile and total leucocyte count being constantly high, to prevent the further progress of the condition and sepsis, wound debridement was planned. Disarticulation of the gangrenous 4 th toe with wide wound debridement was done under spinal anesthesia on 27/01/16. Antibiotics based on culture sensitivity report were administered for 7 days. Systemic symptoms such as fever, nausea, vomiting also subsided. Post procedure there was a considerable reduction in total count. To keep the wound site free from excessive discharge and soaking which would otherwise hamper the healing, VAC (Vacuum assisted wound closure) [7] dressing was done for 9 days. During this course Vrana shodhana (wound cleansing) [8] and vrana ropana (wound healing) [9] drugs were used along with insulin (Mixtard) and oral hypoglycemic (Glycephage) drugs. After vacuum dressing, for about 25 days wound care was done with Thriphala quatha Vrana Parisheka [6] (wound wash) and Jathyadi taila dressing (medicated oil) [9] . Manjistadi kshara vasti [10] as yoga vasti (medicated enema for 8 days) was started on 02/03/16. Skin grafting was proposed after ensuring proper approximation of the wound with healthy granulation [11] . On 05/03/15 a skin flap from right thigh was taken and grafted over the wound under spinal anesthesia under suitable antibiotic coverage. Also, K-wire fixation of the 3rd toe was done to support the loosely attached (after the disarticulation of the 4th toe) distal phalanx. Subsequent dressing showed the skin graft had taken up well and hence discharge was planned. Previous oral medications and insulin were continued. During his stay, other associated complaints such as cough, difficulty in breathing and sleeplessness were managed symptomatically. Patient was advised to continue the same internal medications for a period of 1 month along with daily dressing until the complete healing.
Table 1
Intervention.
| Intervention | Ingredients | Dose | Anupana | Duration |
|---|---|---|---|---|
| Oral medications | ||||
| Kashaya of fresh drugs of Nagara + Amrutha + Haritaki in (1:3:2 proportion) | 60 ml-60 ml-60 ml 1 h before food | – | 3 months | |
| Triphala, pippali, guggulu | 1-1-1 1 h after food | Warm water | 3 months | |
| Shudda Gandhaka, Chaturjatha, guduchi, triphala, shunti, bringaraja, sita, go ksheera | 1-1-1 1 h after food | Warm water | 3 months | |
| procedures | Method of preparation | Method of administration | ||
| Triphala kwatha- 400 ml, gomutra- 100 ml, Madhu- 60 ml, Yava kshara 2 g. Manjistadi taila was used for anuvasana | Given with vasti yantra | 8 days in yoga vasti pattern | ||
| External treatments | ||||
| Avagaha and prakshalana with Triphala kwatha | For 1 part of dry drug 4 parts of water is added and reduced to 1/4th part | For immersion of the affected foot and for washing the wound respectively | 25 days | |
5. Follow-up and outcomes
Patient was followed once a month for 4 months ( Table 2 ). The skin graft was successful with complete wound healing. There was no complaint of pain, discharge or any fresh wound.
Table 2
Time line of events.
| Date | Findings | Intervention | Outcome |
|---|---|---|---|
| 23/01/2016 to 27/01/2016 | Patient visited and diagnosed as | internal and external treatments started (Table 1) | No improvement in wound |
| 27/01/2016 to 28/01/2018 | Patient was continuously febrile and TC constantly high (15,400 to 26,500) | ||
| 28/01/2018 to 30/01/2018 | Wound healthy | external and internal interventions along with antibiotics and anti-diabetics continued | |
| 30/01/2016 | Decision to VAC dressing in order to reduce the wound discharge and assist the quick healing | treatment continued | Successful VAC dressing |
| 30/01/2018 to 08/02/2016 | Wound with healthy granulation tissue filling from beneath | and antidiabetic medications continued | |
| 09/02/2018 to 04/03/2018 | • Satisfactory wound healing | wound care internally and externally | Wound ready for grafting |
| 05/03/16 to 12/03/2018 | Wound margins healthy and floor filled with healthy granulation tissue | Successful grafting | |
| 12/03/2018 to 18/03/2016 | Graft was accepted well |
6. Discussion
The case was managed according to Ayurveda guidelines on different types of wound management along with the use of conventional medicine. Both Ayurvedic and Allopathy science have advantages and disadvantages. Best of each science has been adopted for the better outcome in an integrative manner; hence the disadvantages of each science are left out from the discussion. In the present case the patient was administered the spinal anesthesia to perform the indicated Ayurvedic and Allopathic surgical interventions like incision, excision, wound debridement and skin grafting. This has helped to liberally and adequately handle the tissues without compromising the necessities for a healthy wound healing.
Absence of discharge and maintenance of wound in a dry state consistently is of prime importance in wound healing [12] . Vacuum dressing is one such method to achieve this status where it uses a controlled suction pressure to remove the discharge from the wound [13] . This method assures the surgeon for its better outcome [ Fig. 1 ].

Stagewise pictures of the wound during intervention. A – Wound on first day before treatment; B – Wound after debridement; C – VAC after wound debridement; D – Wound after VAC dressing; E – 15 days after VAC dressing; F – Wound just before skin grafting; G – After 7 days od skin graft; H – At the time of discharge; I – After one month of discharge; J – After 6 months of discharge.
Parisheka (wound wash) helps in removal of the debris from the wound [14] . Triphala kwatha was used for this purpose which helps in removal of the discharge and also cleanses the wound, removes the slough and assists in wound healing ( ropana ) [9] . Medicated enema is indicated in chronic ulcers of lower limbs and helpful in reducing the pain [5] . Studies have shown the efficacy of Jatyadi taila in wound healing [15] . Jatyadi taila cleanses the wound and promotes wound healing. It is indicated in different types of ulcers including those due to injury, bites and chemicals or toxins [16] . Among the internal medications Triphala Guggulu removes the slough from the suppurated wound along with the foul smell. It also helps in reduction of swelling and pain [14] . Gandhaka rasayana helps in removal of the slough, cleansing and healing of the wound. It is indicated in vaataraktaja vrana (ulcer due to peripheral vascular conditions), kushta (skin conditions), prameha (diabetic ulcers) [17] . Amruthotharam kashayam acts as deepana , pachana , lekhana , pakahara , shophahara (anti-inflammatory properties) and rakta prasadana (promotes blood supply) [18] .
Thus, the combination of internal and external management was adequate in helping the wound to heal well.
7. Conclusion
The current integrative approach of adopting both Ayurvedic and Allopathy science along with advanced technique for maintaining the dry state of wound was helpful in managing the diabetic foot without undergoing a major amputation. This poses an interest in further evaluating whether this kind of integrative approaches could give new ray of hope for managing different types of chronic non-healing ulcers.
Patient perspective
Patient was satisfied to have improved without necessitating amputation.
Informed consent
Written permission for publication of this case study had been obtained from the patient.
Sources of funding
Not declared.
Conflict of interest
Peer review under responsibility of Transdisciplinary University, Bangalore.
Pardon Our Interruption
As you were browsing something about your browser made us think you were a bot. There are a few reasons this might happen:
- You've disabled JavaScript in your web browser.
- You're a power user moving through this website with super-human speed.
- You've disabled cookies in your web browser.
- A third-party browser plugin, such as Ghostery or NoScript, is preventing JavaScript from running. Additional information is available in this support article .
To regain access, please make sure that cookies and JavaScript are enabled before reloading the page.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Novel Supramolecular Hydrogel for Infected Diabetic Foot Ulcer Treatment
Affiliations.
- 1 Department of Orthopaedics, The Second Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230601, China.
- 2 Department of Anaesthesia, The First Affiliated Hospital of Anhui Medical University North district, Anhui Public Health Clinical Center, Hefei, Anhui, 230011, China.
- 3 School of Life Sciences, Anhui Medical University, Hefei, Anhui, 230032, China.
- PMID: 39225408
- DOI: 10.1002/adhm.202402092
Multifunctional responsive hydrogels hold significant promise for diabetic foot ulcer (DFU) treatment, though their complex design and manufacturing present challenges. This study introduces a novel supramolecular guanosine-phenylboronic-chlorogenic acid (GBC) hydrogel developed using a dynamic covalent strategy. The hydrogel forms through guanosine quadruplex assembly in the presence of potassium ions and chlorogenic acid (CA) linkage via dynamic borate bonds. GBC hydrogels exhibit pH and glucose responsiveness, releasing more chlorogenic acid under acidic and high glucose conditions due to borate bond dissociation and G-quadruplex (G4) hydrogel disintegration. Experimental results indicate that GBC hydrogels exhibit good self-healing, shear-thinning, injectability, and swelling properties. Both in vitro and in vivo studies demonstrate the GBC hydrogel's good biocompatibility, ability to eliminate bacteria and reactive oxygen species (ROS), facilitate macrophage polarization from the M1 phenotype to the M2 phenotype (decreasing CD86 expression and increasing CD206 expression), exhibit anti-inflammatory effects (reducing TNF-α expression and increasing IL-10 expression), and promote angiogenesis (increasing VEGF, CD31, and α-SMA expression). Thus, GBC hydrogels accelerate DFU healing and enhance tissue remodeling and collagen deposition. This work provides a new approach to developing responsive hydrogels to expedite DFU healing.
Keywords: angiogenesis enhancement; antibacterial properties; diabetic foot ulcers; immunomodulation; ph/glucose dual responsive hydrogels.
© 2024 Wiley‐VCH GmbH.
PubMed Disclaimer
- A. Misra, H. Gopalan, R. Jayawardena, A. P. Hills, M. Soares, A. A. Reza‐Albarran, K. L. Ramaiya, J. Diabetes 2019, 11, 522.
- E. Standl, K. Khunti, T. B. Hansen, O. Schnell, Eur. J. Prev. Cardiol. 2019, 26, 7.
- J. J. Hurlow, G. J. Humphreys, F. L. Bowling, A. J. McBain, Int. Wound J. 2018, 15, 814.
- X. Hu, J. He, L. Qiao, C. Wang, Y. Wang, R. Yu, W. Xu, F. Wang, S. Yang, X. Zhang, Z. Qian, Adv. Funct. Mater. 2024, 34, 2312140.
- Y. K. Wu, N. C. Cheng, C. M. Cheng, Trends Biotechnol. 2019, 37, 505.
Grants and funding
- 2208085MH223/Natural Science Foundation of Anhui Province
- 2022AH050784/Key projects of Natural Science Foundation of Universities in Anhui Province
- 2022xkj200/Anhui Medical University Foundation
- 52003006/National Natural Science Foundation of China
- AHWJ2023A20126/Scientific Research Foundation of Education Department of Anhui Province of China
LinkOut - more resources
Full text sources.

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

IMAGES
VIDEO
COMMENTS
Mariam TG, Alemayehu A, Tesfaye E, et al. Prevalence of diabetic foot ulcer and associated factors among adult diabetic patients who attend the diabetic follow-up clinic at the University of Gondar Referral Hospital, North West Ethiopia, 2016: institutional-based cross-sectional study.
In case 1, a 56-year-old man with 3-years history of type 2 diabetes had a 3.0×2.0 cm ulcer which infected with staphylococcus aureus on his right calf for more than half a month. In case 2, a 70-year-old man with 10-years history of type 2 diabetes presented with an 8-month right heel ulcer that developed to 7.5×4.6 cm.
ABSTRACT. Description of a clinical case of a long-standing diabetic patient, who on admission to prison presents ulcerations on both feet, of ten years of evolution. Until his admission to the Penitentiary Center he has suffered repeated hospital admissions, repeatedly proposing amputation, which the patient refused.
Figure 1 Imaging diagnosis of diabetic lower extremity ulcers before treatment for case 1 (A-C) and case 2 (D-F).(A) Picture showed an ulcerated surface on the inner skin of the right calf, about 3.0×2.0 cm in size and 6 mm in depth; (B) MRI showed a local subcutaneous soft tissue defect at the medial margin of the right calf, with swelling in the margin and adjacent soft tissue space; (C ...
Abstract. Diabetic foot ulceration is a devastating complication of diabetes that is associated with infection, amputation, and death, and is affecting increasing numbers of patients with diabetes mellitus. The pathogenesis of foot ulcers is complex, and different factors play major roles in different stages. The refractory nature of foot ulcer ...
This case report presents the case study of a male patient with an 8-year history of diabetes who developed a diabetic foot ulcer. The patient was undergoing treatment with metformin and insulin. Despite the severity of the ulcer, a combination therapy approach, including surgical debridement, maggot therapy, and negative pressure wound therapy ...
Angirasa A, Willrich A, Cooper B, Stuck R. Combining bioengineered human dermal replacement and multilayered compression dressings to manage ulcers in a person with diabetes mellitus. A case study. Osteotomy Wound Manage. 2006;52(5):60-64. Atkin L, Tansley J, Stephenson J. Diabetic foot ulcers: The impact of oedema. Wounds UK. 2018;14(1):41-8.
A multidisciplinary team (MDT) approach is the most efficient way to treat many chronic and serious diseases. In this case report, providers sought to implement an MDT approach to treat a patient with diabetes and foot ulcers, actively involving the patient's caregiving family members. Comprehensive evaluation, blood sugar control, and timely ...
Case studies 4-6: diabetic foot ulcers J Wound Care. 2022 Aug 1;31(Sup8a):S18-S21. doi: 10.12968/jowc.2022.31.Sup8a.S18. Author Kimberley Wilde 1 Affiliation 1 Advanced Podiatrist, Manchester University NHS Foundation Trust, UK. PMID: 35994427 DOI: 10.12968 ...
This article explores local barriers to diabetic foot ulcer healing, and describes the use of a dressing designed to manage exudate, infection and biofilm (AQUACEL® Ag+ dressing (AQAg+)) on recalcitrant diabetic foot ulcers. The authors consider four case studies that demonstrate how managing local barriers to wound healing with antimicrobial ...
Introduction. The term diabetes-related foot ulcer (DFU) has been defined as a break in the skin of the foot of a person with diabetes, which penetrates as a minimum to the epidermis and part of the dermis. 1 An ulcer might be triggered by trauma—whether from an accident or from the effect of excessive local forces—or its precipitating cause might not be clear.
481. DIABETIC FOOT ULCER: A CASE REPORT. *Shraddha Devarshi and Vinod Kumar 1. Department of Clinical Pharmacy (Phar m.D), Poona College of Pharmacy, Bharati Vidyapeeth Deemed Universit y, Pune ...
This study was sponsored by the author and approved for clinical investigation by the Sterling Investigational Review Board, Atlanta, Georgia (#6606-001). ... Novitasari D, Amin N, Minulo TT. Risk factors for lower extremity amputation in patients with diabetic foot ulcers: a hospital-based-case-control study. Diabet Foot Ankle 2015;6:296-9 ...
Case study: Diabetic ulcer on foot Contributed by Dr. Jeffery Lehrman, DPM, FACFAS Patient 89-year-old Caucasian male living with a history of non-insulin-dependent diabetes mellitus (NIDDM). Wound presentation • Ulcer on medial right hallux interphalangeal joint, noticed four days
Case Study: Treating A Patient With A Chronic Diabetic Foot Ulcer. This author navigates the complex issues in treating an elderly patient with a heavily exudative diabetic foot ulcer, which has recurred over an 11-year period. This case is compelling for several reasons. It illustrates the importance of the team approach to limb salvage in a ...
Diabetes & Primary Care's series of interactive case studies is aimed at all healthcare professionals in primary and community care who would like to broaden their understanding of diabetes.. Around one in three people with diabetes will develop a foot ulcer within their lifetime. Primary care plays a critical role in identifying problems with the diabetic foot, and in responding rapidly and ...
The study revealed that diabetic foot ulcer was more prevalent among male patients with type 2 diabetes since 11 to 25 yrs belongs to the age group between 51-60 years.
Hi, everyone. We're going to go through a case study for a diabetic foot ulcer together. Let's get started. In this scenario, Michael is a 15-year-old male diagnosed last year with diabetes type one. He presents to the acute care unit with a sore that will not heal on the bottom of his right foot. He states that he sees an endocrinologist for ...
Here, we present the case of a 57-year-old man with type 1 diabetes mellitus and a chronic foot ulcer who was successfully managed with a remote patient-facing wound care smart-phone application. The use of an innovative patient-facing smart phone app designed to monitor and manage wounds by a patient with diabetes and foot ulcer resulted in ...
As metformin can accelerate wound healing, this could be welcome news for the 18.6 million people worldwide (1.6 million in the U.S.) who develop a diabetic foot ulcer, or DFU, in their lifetimes.
Plantar shear stress may have an important role in the formation of a Diabetic Foot Ulcer, but its measurement is regarded as challenging and has limited research. This paper highlights the importance of anatomical specific shear sensor calibration and presents a feasibility study of a novel shear sensing system which has measured in-shoe shear stress from gait activity on both healthy and ...
After proving acellular matrices are effective on diabetic foot ulcers, researchers are eyeing the treatment for venous leg ulcers. ... repeated violations' in $375K overtime non-payment case.
Aim: To compare the effectiveness of preventive interventions in reducing reccurrent diabetic foot ulcers. Meta-analysis (MA) was conducted to address clinical questions on this topic of the Italian guidelines on diabetic foot. Methods: This MA includes randomized controlled trials evaluating the effectiveness of various preventive interventions, namely: treatment of pre-ulcerative foot ...
INTRODUCTION. Diabetic foot ulcers (DFU) are common clinical problems and devastating complications of diabetes, and affect 15% of all diabetic patients and results in significant morbidity, mortality, and financial burdens[].Five-year risk of mortality for a patient with diabetic foot ulcer is 2.5 times higher than the risk for a patient without[].
Diabetic foot ulcers (DFUs) present a significant health challenge, demanding innovative solutions for timely identification and evaluation. This study introduces "SoleScan," a novel approach to revolutionize DFU management. Leveraging advanced technologies, including machine learning and computer vision, SoleScan endeavors to improve the precision and effectiveness of DFU identification. The ...
What to Do When a Diabetic Attack or Emergency Strikes
Non-healing diabetic foot has always demanded more attention from the surgeon due to the distinct problem it possesses. There is a constant need for the evolvement in the management and this case is one such attempt. A 62-year-old male patient with a history of diabetes mellitus since 10 years presented with a non-healing foot ulcer since 1 month.
The aim of the study is to elucidate community nurses' professional basis for treating diabetic foot ulcers. A situational case study design was adopted in an archetypical Danish community nursing setting. Experience is a crucial component in the community nurses' professional basis for treating diabetic foot ulcers.
Page | 2 For this case we will be looking into Ms. Craft case where she was diagnosed with what is called decompensated diabetes mellitus. Diabetes Mellitus is defined as a disease that will affect how the body uses blood sugar (Mayo Clinic Staff , 2023). Also, metabolic acidosis is a condition where acid buildups in the body which stems from diabetes being left untreated.
Multifunctional responsive hydrogels hold significant promise for diabetic foot ulcer (DFU) treatment, though their complex design and manufacturing present challenges. This study introduces a novel supramolecular guanosine-phenylboronic-chlorogenic acid (GBC) hydrogel developed using a dynamic cova …