Featured Topics
Featured series.
A series of random questions answered by Harvard experts.

Explore the Gazette
Read the latest.

Loving your pup may be a many splendored thing

Aspirin may help cut colorectal cancer risk
Faster ‘in a dish’ model may speed up treatment for Parkinson’s
Estrogen a more powerful breast cancer culprit than we realized.
Getty Images
Ekaterina Pesheva
HMS Communications
Potential path to better testing in findings that identify hormone as ‘a catalyst and a cause’ in disease
In what may turn out to be a long-missing piece in the puzzle of breast cancer, Harvard Medical School researchers have identified the molecular sparkplug that ignites cases of the disease currently unexplained by the classical model of breast-cancer development.
A report on the team’s work is published May 17 in Nature.
“We have identified what we believe is the original molecular trigger that initiates a cascade culminating in breast tumor development in a subset of breast cancers that are driven by estrogen,” said study senior investigator Peter Park , professor of Biomedical Informatics in the Blavatnik Institute at HMS.
The researchers said as many as one-third of breast cancer cases may arise through the newly identified mechanism.
The study also shows that the sex hormone estrogen is the culprit behind this molecular dysfunction because it directly alters a cell’s DNA.
Most, though not all, breast cancers are fueled by hormonal fluctuations . The prevailing view of estrogen’s role in breast cancer is that it acts as a catalyst for cancer growth because it stimulates the division and proliferation of breast tissue, a process that carries the risk for cancer-causing mutations. The new work, however, shows that estrogen causes mischief in a far more direct manner.
“Our work demonstrates that estrogen can directly induce genomic rearrangements that lead to cancer, so its role in breast cancer development is both that of a catalyst and a cause,” said study first author Jake Lee , a former research fellow in the Park lab who is now a medical oncology fellow at Memorial Sloan Kettering Cancer Center.
Although the work has no immediate implications for therapy, it could inform the design of tests that can track treatment response and could help doctors detect the return of tumors in patients with a history of certain breast cancers.
Birth of a cancer cell
The human body is made up of hundreds of trillions of cells. Most of these cells are constantly dividing and replicating, a process that sustains the function of organs day after day, over a lifetime.
With each division, a cell makes a copy of its chromosomes — bundles of tightly compressed DNA — into a new cell. But this process sometimes goes awry, and DNA can break. In most cases, these DNA breaks get swiftly mended by the molecular machinery that guards the integrity of the genome. However, every now and then, the repair of broken DNA gets botched, causing chromosomes to get misplaced or scrambled inside a cell.
Many human cancers arise in this manner during cell division, when chromosomes get rearranged and awaken dormant cancer genes that can trigger tumor growth.
One such chromosomal scramble can occur when a chromosome breaks, and a second copy of the broken chromosome is made before the break gets fixed.
Then, in what ends up being a botched repair attempt, the broken end of one chromosome is fused to the broken end of its sister copy rather than to its original partner. The resulting new structure is a misshapen, malfunctioning chromosome.
During the next cell division, the misshapen chromosome is stretched between the two emerging daughter cells and the chromosome “bridge” breaks, leaving behind shattered fragments that contain cancer genes to multiply and get activated.
“Our work demonstrates that estrogen can directly induce genomic rearrangements that lead to cancer, so its role in breast cancer development is both that of a catalyst and a cause.” Jake Lee, medical oncology fellow at Memorial Sloan Kettering Cancer Center
Certain human cancers, including some breast cancers, arise when a cell’s chromosomes get rearranged in this way. This malfunction was first described in the 1930s by Barbara McClintock , who went on to win the Nobel Prize in physiology or medicine in 1983.
Cancer experts can often identify this particular aberration in tumor samples by using genomic sequencing. Yet, a portion of breast cancer cases do not harbor this mutational pattern, raising the question: What is causing these tumors?
These were the “cold” cases that intrigued study authors Park and Lee. Looking for answers, they analyzed the genomes of 780 breast cancers obtained from patients diagnosed with the disease. They expected to find the classical chromosomal disarray in most of the tumor samples, but many of the tumor cells bore no trace of this classic molecular pattern.
Instead of the classic misshapen and improperly patched-up single chromosome, they saw that two chromosomes had fused, suspiciously near “hot spots” where cancer genes are located.
Just as in McClintock’s model, these rearranged chromosomes had formed bridges, except in this case, the bridge contained two different chromosomes. This distinctive pattern was present in one-third (244) of the tumors in their analysis.
Lee and Park realized they had stumbled upon a new mechanism by which a “disfigured” chromosome is generated and then fractured to fuel the mysterious breast cancer cases.
A new role for estrogen in breast cancer?
When the researchers zoomed onto the hot spots of cancer-gene activation, they noticed that these areas were curiously close to estrogen-binding areas on the DNA.
Estrogen receptors are known to bind to certain regions of the genome when a cell is stimulated by estrogen. The researchers found that these estrogen-binding sites were frequently next to the zones where the early DNA breaks took place.
This offered a strong clue that estrogen might be somehow involved in the genomic reshuffling that gave rise to cancer-gene activation.
Lee and Park followed up on that clue by conducting experiments with breast cancer cells in a dish. They exposed the cells to estrogen and then used CRISPR gene editing to make cuts to the cells’ DNA.
As the cells mended their broken DNA, they initiated a repair chain that resulted in the same genomic rearrangement Lee and Park had discovered in their genomic analyses.
Estrogen is already known to fuel breast cancer growth by promoting the proliferation of breast cells. However, the new observations cast this hormone in a different light.
They show estrogen is a more central character in cancer genesis because it directly alters how cells repair their DNA.
The findings suggest that estrogen-suppressing drugs such as tamoxifen — often given to patients with breast cancer to prevent disease recurrence — work in a more direct manner than simply reducing breast cell proliferation.
“In light of our results, we propose that these drugs may also prevent estrogen from initiating cancer-causing genomic rearrangements in the cells, in addition to suppressing mammary cell proliferation,” Lee said.
The study could lead to improved breast cancer testing. For instance, detecting the genomic fingerprint of the chromosome rearrangement could alert oncologists that a patient’s disease is coming back, Lee said.
A similar approach to track disease relapse and treatment response is already widely used in cancers that harbor critical chromosomal translocations, including certain types of leukemias.
More broadly, the work underscores the value of DNA sequencing and careful data analysis in deepening the biology of cancer development, the researchers said.
“It all started with a single observation. We noticed that the complex pattern of mutations that we see in genome sequencing data cannot be explained by the textbook model,” Park said. “But now that we’ve put the jigsaw puzzle together, the patterns all make sense in light of the new model. This is immensely gratifying.” Additional authors included Youngsook Lucy Jung, Taek-Chin Cheong, Jose Espejo Valle-Inclan, Chong Chu, Doga C. Gulhan,Viktor Ljungstrom, Hu Jin, Vinayak Viswanadham, Emma Watson, Isidro Cortes-Ciriano, Stephen Elledge, Roberto Chiarle, and David Pellman.
This work was funded by grants from Ludwig Center at Harvard, Cancer Grand Challenges, Cancer Research UK, and the Mark Foundation for Cancer Research, National Institutes of Health grant 1R01-CA222598, and with additional support from the Office of Faculty Development/CTREC/BTREC Career Development Fellowship.
Share this article
You might like.

New research suggests having connection to your dog may lower depression, anxiety

New research suggests those with less healthy lifestyles may get highest benefit from regular use

Could result in personalized models to test diagnostic and treatment strategies
Good genes are nice, but joy is better
Harvard study, almost 80 years old, has proved that embracing community helps us live longer, and be happier
Committee named to lead Legacy of Slavery memorial project
University names committee to lead Harvard & the Legacy of Slavery Memorial Project.
NCI study advances personalized immunotherapy for metastatic breast cancer
- Posted: February 1, 2022
240-760-6600
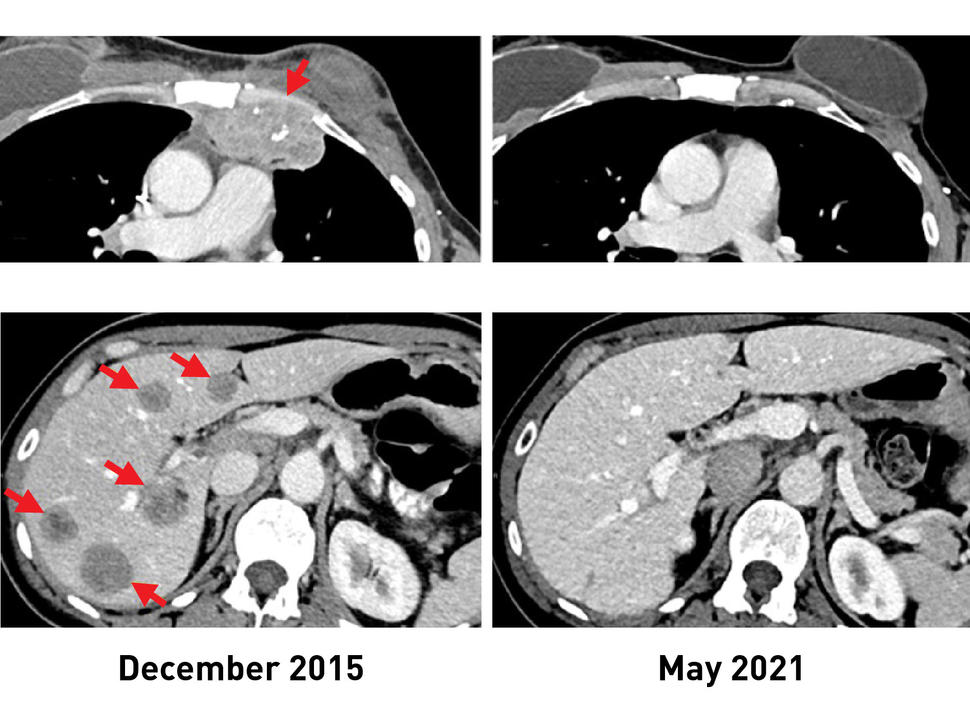
Before TIL therapy, a woman with breast cancer had metastatic lesions in her chest wall (top, left) and liver (bottom, left). After receiving the immunotherapy, her tumors shrank completely, and recent scans (right) show that she remains cancer free more than 5 years later.
An experimental form of immunotherapy that uses an individual’s own tumor-fighting immune cells could potentially be used to treat people with metastatic breast cancer, according to results from an ongoing clinical trial led by researchers at the National Cancer Institute’s (NCI) Center for Cancer Research, part of the National Institutes of Health. Many people with metastatic breast cancer can mount an immune reaction against their tumors, the study found, a prerequisite for this type of immunotherapy, which relies on what are called tumor-infiltrating lymphocytes (TILs).
In a clinical trial of 42 women with metastatic breast cancer, 28 (or 67%) generated an immune reaction against their cancer. The approach was used to treat six women, half of whom experienced measurable tumor shrinkage. Results from the trial appeared Feb. 1, 2022, in the Journal of Clinical Oncology .
“It’s popular dogma that hormone receptor–positive breast cancers are not capable of provoking an immune response and are not susceptible to immunotherapy,” said study leader Steven A. Rosenberg, M.D., Ph.D., chief of the Surgery Branch in NCI’s Center for Cancer Research. “The findings suggest that this form of immunotherapy can be used to treat some people with metastatic breast cancer who have exhausted all other treatment options.”
Immunotherapy is a treatment that helps a person’s own immune system fight cancer. However, most available immunotherapies, such as immune checkpoint inhibitors, have shown limited effectiveness against hormone receptor–positive breast cancers, which are the majority of breast cancers.
The immunotherapy approach used in the trial was pioneered in the late 1980s by Dr. Rosenberg and his colleagues at NCI. It relies on TILs, T cells that are found in and around the tumor.
TILs can target tumor cells that have specific proteins on their surface, called neoantigens, that the immune cells recognize. Neoantigens are produced when mutations occur in tumor DNA. Other forms of immunotherapy have been found to be effective in treating cancers, such as melanoma, that have many mutations, and therefore many neoantigens. Its effectiveness in cancers that have fewer neoantigens, such as breast cancer, however, has been less clear.
The results of the new study come from an ongoing phase 2 clinical trial being carried out by Dr. Rosenberg and his colleagues. This trial was designed to see if the immunotherapy approach could lead to tumor regressions in people with metastatic epithelial cancers, including breast cancer. In 2018, the researchers showed that one woman with metastatic breast cancer who was treated in this trial had complete tumor shrinkage, known as a complete response.
In the trial, the researchers used whole-genome sequencing to identify mutations in tumor samples from 42 women with metastatic breast cancer whose cancers had progressed despite all other treatments. The researchers then isolated TILs from the tumor samples and, in lab tests, tested their reactivity against neoantigens produced by the different mutations in the tumor.
Twenty-eight women had TILs that recognized at least one neoantigen. Nearly all the neoantigens identified were unique to each patient.
“It’s fascinating that the Achilles’ heel of these cancers can potentially be the very gene mutations that caused the cancer,” said Dr. Rosenberg. “Since that 2018 study, we now have information on 42 patients, showing that the majority give rise to immune reactions.”
For the six women treated, the researchers took the reactive TILs and grew them to large numbers in the lab. They then returned the immune cells to each patient via intravenous infusion. All the patients were also given four doses of the immune checkpoint inhibitor pembrolizumab (Keytruda) before the infusion to prevent the newly introduced T cells from becoming inactivated.
After the treatment, tumors shrank in three of the six women. One is the original woman reported in the 2018 study, who remains cancer free to this day. The other two women had tumor shrinkage of 52% and 69% after six months and 10 months, respectively. However, some disease returned and was surgically removed. Those women now have no evidence of cancer approximately five years and 3.5 years, respectively, after their TIL treatment.
The researchers acknowledged that the use of pembrolizumab, which has been approved for some early-stage breast cancers, may raise uncertainties about its influence on the outcome of TIL therapy. However, they said, treatment with such checkpoint inhibitors alone has not led to sustained tumor shrinkage in people with hormone receptor–positive metastatic breast cancer.
Dr. Rosenberg said that with the anticipated opening early this year of NCI’s new building devoted to cell-based therapies, he and his colleagues can begin treating more individuals with metastatic breast cancer as part of the ongoing clinical trial. He noted that this new immunotherapy approach could potentially be used for people with other types of cancer as well.
“We’re using a patient’s own lymphocytes as a drug to treat the cancer by targeting the unique mutations in that cancer,” he said. “This is a highly personalized treatment.”
About the Center for Cancer Research (CCR): CCR comprises nearly 250 teams conducting basic, translational, and clinical research in the NCI intramural program — an environment supporting innovative science aimed at improving human health. CCR’s clinical program is housed at the NIH Clinical Center — the world’s largest hospital dedicated to clinical research. For more information about CCR and its programs, visit ccr.cancer.gov .
About the National Cancer Institute (NCI): NCI leads the National Cancer Program and NIH’s efforts to dramatically reduce the prevalence of cancer and improve the lives of cancer patients and their families, through research into prevention and cancer biology, the development of new interventions, and the training and mentoring of new researchers. For more information about cancer, please visit the NCI website at cancer.gov or call NCI’s contact center, the Cancer Information Service, at 1-800-4-CANCER (1-800-422-6237).
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit nih.gov .

- Adolescent and Young Adult Cancer
- Bile Duct Cancer
- Bladder Cancer
- Brain Cancer
- Breast Cancer
- Cervical Cancer
- Childhood Cancer
- Colorectal Cancer
- Endometrial Cancer
- Esophageal Cancer
- Head and Neck Cancer
- Kidney Cancer
- Liver Cancer
- Lung Cancer
- Mouth Cancer
- Mesothelioma
- Multiple Myeloma
- Neuroendocrine Tumors
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer/Melanoma
- Stomach Cancer
- Testicular Cancer
- Throat Cancer
- Thyroid Cancer
- Prevention and Screening
- Diagnosis and Treatment
- Research and Clinical Trials
- Survivorship

Request an appointment at Mayo Clinic

New study finds triple-negative breast cancer tumors with an increase in immune cells have lower risk of recurrence after surgery
Share this:.
By Kelley Luckstein
A new multicenter, international study suggests that people who have early-stage triple-negative breast cancer (TNBC) and high levels of immune cells within their tumors may have a lower risk of recurrence and better survival rates even when not treated with chemotherapy. The study was published today in the Journal of American Medical Association (JAMA).
TNBC is a breast cancer subtype that does not respond to drugs that target the estrogen receptor or the HER2 protein. It grows rapidly, is more likely to spread beyond the breast before diagnosis and is more likely to recur than other breast cancers. TNBC represents about 15% of all breast cancers and is more common in younger people and in women of African American, Hispanic and Indian descent. Immune cells, also known as tumor-infiltrating lymphocytes, or TILs, are naturally existing immune system cells that can move from the bloodstream into a tumor and can recognize and destroy cancer cells.

"This is an important finding because it highlights that the abundance of TILs in breast tissue is a prognostic biomarker in people with early-stage triple-negative breast cancer, even when chemotherapy is not administered," says Roberto Leon-Ferre, M.D. , a breast medical oncologist at Mayo Clinic Comprehensive Cancer Center and first author of the study. "The study's findings may inspire future clinical trials to explore whether patients with a favorable prognosis (high TILs) can avoid intensive chemotherapy regimens."
"This meta-analysis confirms robustly the prognostic value of TILs that we have previously reported in TNBC patients treated with chemotherapy and expands it to patients treated without chemotherapy," says Sarah Flora Jonas, Ph.D., a statistician at Gustave Roussy and co-first author of the study. "Future studies may allow the use of this biomarker along with standard clinicopathological factors to inform treatment decisions in TNBC patients."
"Of interest, the first report suggesting that an increased number of immune cells being associated with better prognosis in breast cancer patients was described by doctors at Mayo Clinic more than 100 years ago," says Roberto Salgado, M.D., co-chair of the International Immuno-Oncology Biomarker Working Group; co-lead of the study; and pathologist from the Peter MacCallum Cancer Centre, Melbourne, Australia, and ZAS Hospitals, Antwerp, Belgium. "It took a global effort and a century later to reexamine this biomarker and bring it closer to application in patient care."

"TILs are not currently measured or reported in the routine examination of tissue samples of breast cancer," says co-senior author, Matthew Goetz, M.D. , a medical oncologist at Mayo Clinic Comprehensive Cancer Center and the Erivan K. Haub Family Professor of Cancer Research Honoring Richard F. Emslander, M.D. "While prior studies have focused on measuring TILs in people treated with chemotherapy, this is the largest study to comprehensively demonstrate that the presence of TILs influences the natural behavior of breast cancer in people who have surgery and/or radiation with no additional medical treatment."
For this study, Mayo Clinic and Gustave Roussy researchers, in collaboration with the International Immuno-Oncology Biomarker Working Group, led 11 additional groups to collect data on 1,966 participants with early-stage TNBC who only underwent surgery with or without radiation therapy but did not receive chemotherapy. The participants had been followed for a median of 18 years. The results showed that higher levels of TILs in breast cancer tissue were associated with lower recurrence rates among participants with early-stage TNBC.
"Five years after surgery, 95% of participants with small tumors, stage 1 TNBC, and whose tumors had high TILs were alive, compared to 82% of patients whose tumors had low TILs. Importantly, the breast cancer recurrence rate was significantly lower among patients whose tumors had high TILs," says co-senior author, Stefan Michiels, Ph.D. , head of Oncostat team, Gustave Roussy, Inserm U1018, University Paris-Saclay. "With nearly 2,000 participants involved in the study, we have now assembled the largest international cohort across three continents of people with TNBC in which the primary treatment was surgery without chemotherapy."
"The results of this study could lead to a recommendation to include TILs in the pathology reports of early-stage TNBC worldwide, as it has the potential to inform clinicians and patients when they discuss treatment options," says Dr. Salgado.
Furthermore, this biomarker would only require a visual evaluation by a pathologist looking through a microscope, meaning there are no additional costs associated with identifying the presence of immune cells. This could be particularly beneficial to regions with limited resources, adds Dr. Leon-Ferre.
Most people with early-stage TNBC undergo chemotherapy either before or after surgery, including people with stage 1 breast cancer. Most people receive multiple chemotherapy drugs in combination, which can cause significant side effects. Currently, the main factors considered to determine the course of chemotherapy treatment for each person are the tumor size and whether the cancer has spread to the lymph nodes. However, the authors identified that the number of TILs further influences the risk of future recurrence.
The researchers plan to evaluate TILs as biomarkers in prospective clinical trials evaluating chemotherapy selection based on TIL levels. Ongoing efforts to conduct additional research with other potential biomarkers are underway.
For a complete list of authors, disclosures and funding, see the full paper here .
Learn more about breast cancer and find a clinical trial at Mayo Clinic.
Join the Breast Cancer Support Group on Mayo Clinic Connect , an online community moderated by Mayo Clinic for patients and caregivers.
Also, read these articles:
- Understanding triple-negative breast cancer and its treatment
- 17-gene signature linked to remission after triple-negative breast cancer treatment
A version of this article was originally published as a press release on the Mayo Clinic News Network .
Related Posts

Dr. Christin Harless explains the benefits and drawbacks of the different breast reconstruction options following mastectomy.

A breast MRI and a compassionate radiology technologist helped Melissa Neuman get the breast cancer screening she needed to save her life.

Rebecca Kath, a Mayo Clinic physician assistant, explains how 3D tattoos are used to restore the appearance of nipples and areolas.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cancers (Basel)

Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review
Sergiusz Łukasiewicz.
1 Department of Surgical Oncology, Center of Oncology of the Lublin Region St. Jana z Dukli, 20-091 Lublin, Poland; lp.lzoc@zciweisakulS (S.Ł.); [email protected] (A.S.)
Marcin Czeczelewski
2 Department of Forensic Medicine, Medical University of Lublin, 20-090 Lublin, Poland; [email protected] (M.C.); lp.teno@amrofa (A.F.)
Alicja Forma
3 Department of Human Anatomy, Medical University of Lublin, 20-090 Lublin, Poland; [email protected]
Robert Sitarz
Andrzej stanisławek.
4 Department of Oncology, Chair of Oncology and Environmental Health, Medical University of Lublin, 20-081 Lublin, Poland
Simple Summary
Breast cancer is the most common cancer among women. It is estimated that 2.3 million new cases of BC are diagnosed globally each year. Based on mRNA gene expression levels, BC can be divided into molecular subtypes that provide insights into new treatment strategies and patient stratifications that impact the management of BC patients. This review addresses the overview on the BC epidemiology, risk factors, classification with an emphasis on molecular types, prognostic biomarkers, as well as possible treatment modalities.
Breast cancer (BC) is the most frequently diagnosed cancer in women worldwide with more than 2 million new cases in 2020. Its incidence and death rates have increased over the last three decades due to the change in risk factor profiles, better cancer registration, and cancer detection. The number of risk factors of BC is significant and includes both the modifiable factors and non-modifiable factors. Currently, about 80% of patients with BC are individuals aged >50. Survival depends on both stage and molecular subtype. Invasive BCs comprise wide spectrum tumors that show a variation concerning their clinical presentation, behavior, and morphology. Based on mRNA gene expression levels, BC can be divided into molecular subtypes (Luminal A, Luminal B, HER2-enriched, and basal-like). The molecular subtypes provide insights into new treatment strategies and patient stratifications that impact the management of BC patients. The eighth edition of TNM classification outlines a new staging system for BC that, in addition to anatomical features, acknowledges biological factors. Treatment of breast cancer is complex and involves a combination of different modalities including surgery, radiotherapy, chemotherapy, hormonal therapy, or biological therapies delivered in diverse sequences.
1. Introduction
Being characterized by six major hallmarks, carcinogenesis might occur in every cell, tissue, and organ, leading to the pathological alternations that result in a vast number of cancers. The major mechanisms that enable its progression include evasion of apoptosis, limitless capacity to divide, enhanced angiogenesis, resistance to anti-growth signals and induction of own growth signals, as well as the capacity to metastasize [ 1 ]. Carcinogenesis is a multifactorial process that is primarily stimulated by both—genetic predispositions and environmental causes. The number of cancer-related deaths is disturbingly increasing every year ranking them as one of the major causes of death worldwide. Even though a significant number of cancers do not always need to result in death, they significantly lower the quality of life and require larger costs in general.
Breast cancer is currently one of the most prevalently diagnosed cancers and the 5th cause of cancer-related deaths with an estimated number of 2.3 million new cases worldwide according to the GLOBOCAN 2020 data [ 2 ]. Deaths due to breast cancer are more prevalently reported (an incidence rate approximately 88% higher) in transitioning countries (Melanesia, Western Africa, Micronesia/Polynesia, and the Caribbean) compared to the transitioned ones (Australia/New Zealand, Western Europe, Northern America, and Northern Europe). Several procedures such as preventive behaviors in general as well as screening programs are crucial regarding a possible minimization of breast cancer incidence rate and the implementation of early treatment. Currently, it is the Breast Health Global Initiative (BHGI) that is responsible for the preparation of proper guidelines and the approaches to provide the most sufficient breast cancer control worldwide [ 3 ]. In this review article, we have focused on the female breast cancer specifically since as abovementioned, it currently constitutes the most prevalent cancer amongst females.
2. Breast Cancer Epidemiology
According to the WHO, malignant neoplasms are the greatest worldwide burden for women, estimated at 107.8 million Disability-Adjusted Life Years (DALYs), of which 19.6 million DALYs are due to breast cancer. [ 4 ]. Breast cancer is the most frequently diagnosed cancer in women worldwide with 2.26 million [95% UI, 2.24–2.79 million] new cases in 2020 [ 5 ]. In the United States, breast cancer alone is expected to account for 29% of all new cancers in women [ 6 ]. The 2018 GLOBOCAN data shows that age-standardized incidence rates (ASIR) of breast cancer are strongly and positively associated with the Human Development Index (HDI) [ 7 ]. According to 2020 data, the ASIR was the highest in very high HDI countries (75.6 per 100,000) while it was more than 200% lower in medium and low HDI countries (27.8 per 100,000 and 36.1 per 100,000 respectively) [ 5 ].
Besides being the most common, breast cancer is also the leading cause of cancer death in women worldwide. Globally, breast cancer was responsible for 684,996 deaths [95% UI, 675,493–694,633] at an age-adjusted rate of 13.6/100,000 [ 5 ]. Although incidence rates were the highest in developed regions, the countries in Asia and Africa shared 63% of total deaths in 2020 [ 5 ]. Most women who develop breast cancer in a high-income country will survive; the opposite is true for women in most low-income and many middle-income countries [ 8 ].
In 2020 breast cancer mortality-to-incidence ratio (MIR) as a representative indicator of 5-year survival rates [ 9 ] was 0.30 globally [ 5 ]. Taking into consideration the clinical extent of breast cancer, in locations with developed health care (Hong-Kong, Singapore, Turkey) the 5-year survival was 89.6% for localized and 75.4% for regional cancer. In less developed countries (Costa Rica, India, Philippines, Saudi Arabia, Thailand) the survival rates were 76.3% and 47.4% for localized and regional breast cancer respectively [ 10 ].
Breast cancer incidence and death rates have increased over the last three decades. Between 1990 and 2016 breast cancer incidence has more than doubled in 60/102 countries (e.g., Afghanistan, Philippines, Brazil, Argentina), whereas deaths have doubled in 43/102 countries (e.g., Yemen, Paraguay, Libya, Saudi Arabia) [ 11 ]. Current projections indicate that by 2030 the worldwide number of new cases diagnosed reach 2.7 million annually, while the number of deaths 0.87 million [ 12 ]. In low- and medium-income countries, the breast cancer incidence is expected to increase further due to the westernization of lifestyles (e.g., delayed pregnancies, reduced breastfeeding, low age at menarche, lack of physical activity, and poor diet), better cancer registration, and cancer detection [ 13 ].
3. Risk Factors of Breast Cancer
The number of risk factors of breast cancer is significant and includes both modifiable factors and non-modifiable factors ( Table 1 ).
Modifiable and non-modifiable risk factors of breast cancer.
| Non-Modifiable Factors | Modifiable Factors |
|---|---|
| Female sex | Hormonal replacement therapy |
| Older age | Diethylstilbestrol |
| Family history (of breast or ovarian cancer) | Physical activity |
| Genetic mutations | Overweight/obesity |
| Race/ethnicity | Alcohol intake |
| Pregnancy and breastfeeding | Smoking |
| Menstrual period and menopause | Insufficient vitamin supplementation |
| Density of breast tissue | Excessive exposure to artificial light |
| Previous history of breast cancer | Intake of processed food |
| Non-cancerous breast diseases | Exposure to chemicals |
| Previous radiation therapy | Other drugs |
3.1. Non-Modifiable Factors
3.1.1. female sex.
Female sex constitutes one of the major factors associated with an increased risk of breast cancer primarily because of the enhanced hormonal stimulation. Unlike men who present insignificant estrogen levels, women have breast cells which are very vulnerable to hormones (estrogen and progesterone in particular) as well as any disruptions in their balance. Circulating estrogens and androgens are positively associated with an increased risk of breast cancer [ 14 ]. The alternations within the physiological levels of the endogenous levels of sex hormones result in a higher risk of breast cancer in the case of premenopausal and postmenopausal women; these observations were also supported by the Endogenous Hormones and Breast Cancer Collaborative Group [ 15 , 16 , 17 ].
Less than 1% of all breast cancers occur in men. However, breast cancer in men is a rare disease that’s at the time of diagnosis tends to be more advanced than in women. The average age of men at the diagnosis is about 67. The important factors increase a man’s risk of breast cancer are: older age, BRCA2/BRCA1 mutations, increased estrogen levels, Klinefelter syndrome, family history of breast cancer, and radiation exposure [ 18 ].
3.1.2. Older Age
Currently, about 80% of patients with breast cancer are individuals aged >50 while at the same time more than 40% are those more than 65 years old [ 19 , 20 , 21 ]. The risk of developing breast cancer increases as follows—the 1.5% risk at age 40, 3% at age 50, and more than 4% at age 70 [ 22 ]. Interestingly, a relationship between a particular molecular subtype of cancer and a patient’s age was observed –aggressive resistant triple-negative breast cancer subtype is most commonly diagnosed in groups under 40 age, while in patients >70, it is luminal A subtype [ 21 ]. Generally, the occurrence of cancer in older age is not only limited to breast cancer; the accumulation of a vast number of cellular alternations and exposition to potential carcinogens results in an increase of carcinogenesis with time.
3.1.3. Family History
A family history of breast cancer constitutes a major factor significantly associated with an increased risk of breast cancer. Approximately 13–19% of patients diagnosed with breast cancer report a first-degree relative affected by the same condition [ 23 ]. Besides, the risk of breast cancer significantly increases with an increasing number of first-degree relatives affected; the risk might be even higher when the affected relatives are under 50 years old [ 24 , 25 , 26 ]. The incidence rate of breast cancer is significantly higher in all of the patients with a family history despite the age. This association is driven by epigenetic changes as well as environmental factors acting as potential triggers [ 27 ]. A family history of ovarian cancer—especially those characterized by BRCA1 and BRCA2 mutations—might also induce a greater risk of breast cancer [ 28 ].
3.1.4. Genetic Mutations
Several genetic mutations were reported to be highly associated with an increased risk of breast cancer. Two major genes characterized by a high penetrance are BRCA1 (located on chromosome 17) and BRCA2 (located on chromosome 13). They are primarily linked to the increased risk of breast carcinogenesis [ 29 ]. The mutations within the above-mentioned genes are mainly inherited in an autosomal dominant manner, however, sporadic mutations are also commonly reported. Other highly penetrant breast cancer genes include TP53 , CDH1 , PTEN , and STK11 [ 30 , 31 , 32 , 33 , 34 ]. Except for the increased risk of breast cancer, carriers of such mutations are more susceptible to ovarian cancer as well. A significant number of DNA repair genes that can interact with BRCA genes including ATM , PALB2 , BRIP1 , or CHEK2 , were reported to be involved in the induction of breast carcinogenesis; those are however characterized by a lower penetrance (moderate degree) compared to BRCA1 or BRCA2 ( Table 2 ) [ 29 , 35 , 36 , 37 , 38 ]. According to quite recent Polish research, mutations within the XRCC2 gene could also be potentially associated with an increased risk of breast cancer [ 39 ].
Major genes associated with an increased risk of breast cancer occurrence.
| Penetration | Gene | Chromosome Location | Associated Syndromes/Disorders | Major Functions | Breast Cancer Risk | Ref. |
|---|---|---|---|---|---|---|
| 17q21.31 | Breast cancer Ovarian cancer Pancreatic cancer Fanconi anemia | DNA repair Cell cycle control | 45–87% | [ ] | ||
| 13q13.1 | Breast cancer Ovarian cancer Pancreatic cancer Prostate cancer Fallopian tube cancer Biliary cancer Melanoma Fanconi anemia Glioblastoma Medulloblastoma Wilms tumor | DNA repair Cell cycle control | 50–85% | [ ] | ||
| 17p13.1 | Breast cancer Colorectal cancer Hepatocellular carcinoma Pancreatic cancer Nasopharyngeal carcinoma Li-Fraumeni syndrome Osteosarcoma Adrenocortical carcinoma | DNA repair Cell cycle control Induction of apoptosis Induction of senescence Maintenance of cellular metabolism | 20–40% (even up to 85%) | [ ] | ||
| 16q22.1 | Breast cancer Ovarian cancer Endometrial carcinoma Gastric cancer Prostate cancer | Regulation of cellular adhesions Control of the epithelial cells (proliferation and motility) | 63–83% | [ ] | ||
| 10q23.31 | Breast cancer Prostate cancer Autism syndrome Cowden syndrome 1 Lhermitte-Duclos syndrome | Cell cycle control | 50–85% | [ ] | ||
| 19p13.3 | Breast cancer Pancreatic cancer Testicular tumor Melanoma Peutz-Jeghers syndrome | Cell cycle control Maintenance of energy homeostasis | 32–54% | [ ] | ||
| 11q22.3 | Breast cancer Lymphoma T-cell prolymphocytic leukemia Ataxia-teleangiectasia | DNA repair Cell cycle control | 20–60% | [ ] | ||
| 16p12.2 | Breast cancer Pancreatic cancer Fanconi anemia | DNA repair | 33–58% | [ ] | ||
| 17q23.2 | Breast cancer Fanconi anemia | Involvement in the activity | ND | [ ] | ||
| 22q12.1 | Breast cancer Li-Fraumeni syndrome Prostate cancer Osteosarcoma | Cell cycle control | 20–25% | [ ] | ||
| 7q36.1 | Fanconi anemia Premature ovarian failure Spermatogenic failure | DNA repair | ND | [ ] |
3.1.5. Race/Ethnicity
Disparities regarding race and ethnicity remain widely observed among individuals affected by breast cancer; the mechanisms associated with this phenomenon are not yet understood. Generally, the breast cancer incidence rate remains the highest among white non-Hispanic women [ 51 , 52 ]. Contrarily, the mortality rate due to this malignancy is significantly higher among black women; this group is also characterized by the lowest survival rates [ 53 ].
3.1.6. Reproductive History
Numerous studies confirmed a strict relationship between exposure to endogenous hormones—estrogen and progesterone in particular—and excessive risk of breast cancer in females. Therefore, the occurrence of specific events such as pregnancy, breastfeeding, first menstruation, and menopause along with their duration and the concomitant hormonal imbalance, are crucial in terms of a potential induction of the carcinogenic events in the breast microenvironment. The first full-term pregnancy at an early age (especially in the early twenties) along with a subsequently increasing number of births are associated with a reduced risk of breast cancer [ 54 , 55 ]. Besides, the pregnancy itself provides protective effects against potential cancer. However, protection was observed at approximately the 34th pregnancy week and was not confirmed for the pregnancies lasting for 33 weeks or less [ 56 ]. Women with a history of preeclampsia during pregnancy or children born to a preeclamptic pregnancy are at lower risk of developing breast cancer [ 57 ]. No association between the increased breast cancer risk and abortion was stated so far [ 58 ].
The dysregulated hormone levels during preeclampsia including increased progesterone and reduced estrogen levels along with insulin, cortisol, insulin-like growth factor-1, androgens, human chorionic gonadotropin, corticotropin-releasing factor, and IGF-1 binding protein deviating from the physiological ranges, show a protective effect preventing from breast carcinogenesis. The longer duration of the breastfeeding period also reduces the risk of both the ER/PR-positive and -negative cancers [ 59 ]. Early age at menarche is another risk factor of breast cancer; it is possibly also associated with a tumor grade and lymph node involvement [ 60 ]. Besides, the earlier age of the first menstruation could result in an overall poorer prognosis. Contrarily, early menopause despite whether natural or surgical, lowers the breast cancer risk [ 61 ].
3.1.7. Density of Breast Tissue
The density of breast tissue remains inconsistent throughout the lifetime; however, several categories including low-density, high-density, and fatty breasts have been established in clinical practice. Greater density of breasts is observed in females of younger age and lower BMI, who are pregnant or during the breastfeeding period, as well as during the intake of hormonal replacement therapy [ 62 ]. Generally, the greater breast tissue density correlates with the greater breast cancer risk; this trend is observed both in premenopausal and postmenopausal females [ 63 ]. It was proposed that screening of breast tissue density could be a promising, non-invasive, and quick method enabling rational surveillance of females at increased risk of cancer [ 64 ].
3.1.8. History of Breast Cancer and Benign Breast Diseases
Personal history of breast cancer is associated with a greater risk of a renewed cancerous lesions within the breasts [ 65 ]. Besides, a history of any other non-cancerous alternations in breasts such as atypical hyperplasia, carcinoma in situ, or many other proliferative or non-proliferative lesions, also increases the risk significantly [ 66 , 67 , 68 ]. The histologic classification of benign lesions and a family history of breast cancer are two factors that are strongly associated with breast cancer risk [ 66 ].
3.1.9. Previous Radiation Therapy
The risk of secondary malignancies after radiotherapy treatment remains an individual matter that depends on the patient’s characteristics, even though it is a quite frequent phenomenon that arises much clinical concern. Cancer induced by radiation therapy is strictly associated with an individual’s age; patients who receive radiation therapy before the age of 30, are at a greater risk of breast cancer [ 69 ]. The selection of proper radiotherapy technique is crucial in terms of secondary cancer risk—for instance, tangential field IMRT (2F-IMRT) is associated with a significantly lower risk compared to multiple-field IMRT (6F-IMRT) or double partial arcs (VMAT) [ 70 ]. Besides, the family history of breast cancer in patients who receive radiotherapy additionally enhances the risk of cancer occurrence [ 71 ]. However, Bartelink et al. showed that additional radiation (16 Gy) to the tumor bed combined with standard radiotherapy might decrease the risk of local recurrence [ 72 ].
3.2. Modifiable Factors
3.2.1. chosen drugs.
Data from some research indicates that the intake of diethylstilbestrol during pregnancy might be associated with a greater risk of breast cancer in children; this, however, remains inconsistent between studies and requires further evaluation [ 73 , 74 ]. The intake of diethylstilbestrol during pregnancy is associated with an increased risk of breast cancer not only in mothers but also in the offspring [ 75 ]. This relationship is observed despite the expression of neither estrogen nor progesterone receptors and might be associated with every breast cancer histological type. The risk increases with age; women at age of ≥40 years are nearly 1.9 times more susceptible compared to women under 40. Moreover, breast cancer risk increases with greater diethylstilbestrol doses [ 76 ]. Numerous researches indicate that females who use hormonal replacement therapy (HRT) especially longer than 5 or 7 years are also at increased risk of breast cancer [ 77 , 78 ]. Several studies indicated that the intake of chosen antidepressants, mainly paroxetine, tricyclic antidepressants, and selective serotonin reuptake inhibitors might be associated with a greater risk of breast cancer [ 79 , 80 ]. Lawlor et al. showed that similar risk might be achieved due to the prolonged intake of antibiotics; Friedman et al. observed that breast risk is mostly elevated while using tetracyclines [ 81 , 82 ]. Attempts were made to investigate a potential relationship between hypertensive medications, non-steroidal anti-inflammatory drugs, as well as statins, and an elevated risk of breast cancer, however, this data remains highly inconsistent [ 83 , 84 , 85 ].
3.2.2. Physical Activity
Even though the mechanism remains yet undeciphered, regular physical activity is considered to be a protective factor of breast cancer incidence [ 86 , 87 ]. Chen et al. observed that amongst females with a family history of breast cancer, physical activity was associated with a reduced risk of cancer but limited only to the postmenopausal period [ 88 ]. However, physical activity is beneficial not only in females with a family history of breast cancer but also in those without such a history. Contrarily to the above-mentioned study, Thune et al. pointed out more pronounced effects in premenopausal females [ 89 ]. There are several hypotheses aiming to explain the protective role of physical activity in terms of breast cancer incidence; physical activity might prevent cancer by reducing the exposure to the endogenous sex hormones, altering immune system responses or insulin-like growth factor-1 levels [ 88 , 90 , 91 ].
3.2.3. Body Mass Index
According to epidemiological evidence, obesity is associated with a greater probability of breast cancer. This association is mostly intensified in obese post-menopausal females who tend to develop estrogen-receptor-positive breast cancer. Yet, independently to menopausal status, obese women achieve poorer clinical outcomes [ 92 ]. Wang et al. showed that females above 50 years old with greater Body Mass Index (BMI) are at a greater risk of cancer compared to those with low BMI [ 93 ]. Besides, the researchers observed that greater BMI is associated with more aggressive biological features of tumor including a higher percentage of lymph node metastasis and greater size. Obesity might be a reason for greater mortality rates and a higher probability of cancer relapse, especially in premenopausal women [ 94 ]. Increased body fat might enhance the inflammatory state and affects the levels of circulating hormones facilitating pro-carcinogenic events [ 95 ]. Thus, poorer clinical outcomes are primarily observed in females with BMI ≥ 25 kg/m 2 [ 96 ]. Interestingly, postmenopausal women tend to present poorer clinical outcomes despite proper BMI values but namely due to excessive fat volume [ 97 ]. Greater breast cancer risk with regards to BMI also correlates with the concomitant family history of breast cancer [ 98 ].
3.2.4. Alcohol Intake
Numerous evidences confirm that excessive alcohol consumption is a factor that might enhance the risk of malignancies within the gastrointestinal tract; however, it was proved that it is also linked to the risk of breast cancer. Namely, it is not alcohol type but rather the content of alcoholic beverages that mostly affect the risk of cancer. The explanation for this association is the increased levels of estrogens induced by the alcohol intake and thus hormonal imbalance affecting the risk of carcinogenesis within the female organs [ 99 , 100 ]. Besides, alcohol intake often results in excessive fat gain with higher BMI levels, which additionally increases the risk. Other hypotheses include direct and indirect carcinogenic effects of alcohol metabolites and alcohol-related impaired nutrient intake [ 101 ]. Alcohol consumption was observed to increase the risk of estrogen-positive breast cancers in particular [ 102 ]. Consumed before the first pregnancy, it significantly contributes to the induction of morphological alterations of breast tissue, predisposing it to further carcinogenic events [ 103 ].
3.2.5. Smoking
Carcinogens found in tobacco are transported to the breast tissue increasing the plausibility of mutations within oncogenes and suppressor genes ( p53 in particular). Thus, not only active but also passive smoking significantly contributes to the induction of pro-carcinogenic events [ 104 ]. Besides, longer smoking history, as well as smoking before the first full-term pregnancy, are additional risk factors that are additionally pronounced in females with a family history of breast cancer [ 105 , 106 , 107 , 108 ].
3.2.6. Insufficient Vitamin Supplementation
Vitamins exert anticancer properties, which might potentially benefit in the prevention of several malignancies including breast cancer, however, the mechanism is not yet fully understood. Attempts are continually made to analyze the effects of vitamin intake (vitamin C, vitamin E, B-group vitamins, folic acid, multivitamin) on the risk of breast cancer, nevertheless, the data remains inconsistent and not sufficient to compare the results and draw credible data [ 108 ]. In terms of breast cancer, most studies are currently focused on vitamin D supplementation confirming its potentially protective effects [ 109 , 110 , 111 ]. High serum 25-hydroxyvitamin D levels are associated with a lower incidence rate of breast cancer in premenopausal and postmenopausal women [ 110 , 112 ]. Intensified expression of vitamin D receptors was shown to be associated with lower mortality rates due to breast cancer [ 113 ]. Even so, further evaluation is required since data remains inconsistent in this matter [ 108 , 114 ].
3.2.7. Exposure to Artificial Light
Artificial light at night (ALAN) has been recently linked to increased breast cancer risk. The probable causation might be a disrupted melatonin rhythm and subsequent epigenetic alterations [ 115 ]. According to the studies conducted so far, increased exposure to ALAN is associated with a significantly greater risk of breast cancer compared to individuals with lowered ALAN exposure [ 116 ]. Nonetheless, data regarding the excessive usage of LED electronic devices and increased risk of breast cancer is insufficient and requires further evaluation as some results are contradictory [ 116 ].
3.2.8. Intake of Processed Food/Diet
According to the World Health Organization (WHO), highly processed meat was classified as a Group 1 carcinogen that might increase the risk of not only gastrointestinal malignancies but also breast cancer. Similar observations were made in terms of an excessive intake of saturated fats [ 117 ]. Ultra-processed food is rich in sodium, fat, and sugar which subsequently predisposes to obesity recognized as another factor of breast cancer risk [ 118 ]. It was observed that a 10% increase of ultra-processed food in the diet is associated with an 11% greater risk of breast cancer [ 118 ]. Contrarily, a diet high in vegetables, fruits, legumes, whole grains, and lean protein is associated with a lowered risk of breast cancer [ 119 ]. Generally, a diet that includes food containing high amounts of n-3 PUFA, vitamin D, fiber, folate, and phytoestrogen might be beneficial as a prevention of breast cancer [ 120 ]. Besides, lower intake of n-6 PUFA and saturated fat is recommended. Several in vitro and in vivo studies also suggest that specific compounds found in green tea might present anti-cancer effects which has also been studied regarding breast cancer [ 121 ]. Similar properties were observed in case of turmeric-derived curcuminoids as well as sulforaphane (SFN) [ 122 , 123 ].
3.2.9. Exposure to Chemical
Chronic exposure to chemicals can promote breast carcinogenesis by affecting the tumor microenvironment subsequently inducing epigenetic alterations along with the induction of pro-carcinogenic events [ 124 ]. Females chronically exposed to chemicals present significantly greater plausibility of breast cancer which is further positively associated with the duration of the exposure [ 125 ]. The number of chemicals proposed to induce breast carcinogenesis is significant; so far, dichlorodiphenyltrichloroethane (DDT) and polychlorinated biphenyl (PCB) are mostly investigated in terms of breast cancer since early exposure to those chemicals disrupts the development of mammary glands [ 126 , 127 ]. A potential relationship was also observed in the case of increased exposure to polycyclic aromatic hydrocarbons (PAH), synthetic fibers, organic solvents, oil mist, and insecticides [ 128 ].
3.2.10. Other Drugs
Other drugs that might constitute potential risk factors for breast cancer include antibiotics, antidepressants, statins, antihypertensive medications (e.g., calcium channel blockers, angiotensin II-converting enzyme inhibitors), as well as NSAIDs (including aspirin, ibuprofen) [ 129 , 130 , 131 , 132 , 133 ].
4. Breast Cancer Classification
4.1. histological classification.
Invasive breast cancers (IBC) comprise wide spectrum tumors that show a variation concerning their clinical presentation, behavior, and morphology. The World Health Organization (WHO) distinguish at least 18 different histological breast cancer types [ 134 ].
Invasive breast cancer of no special type (NST), formerly known as invasive ductal carcinoma is the most frequent subgroup (40–80%) [ 135 ]. This type is diagnosed by default as a tumor that fails to be classified into one of the histological special types [ 134 ]. About 25% of invasive breast cancers present distinctive growth patterns and cytological features, hence, they are recognized as specific subtypes (e.g., invasive lobular carcinoma, tubular, mucinous A, mucinous B, neuroendocrine) [ 136 ].
Molecular classification independently from histological subtypes, invasive breast cancer can be divided into molecular subtypes based on mRNA gene expression levels. In 2000, Perou et al. on a sample of 38 breast cancers identified 4 molecular subtypes from microarray gene expression data: Luminal, HER2-enriched, Basal-like, and Normal Breast-like [ 137 ]. Further studies allowed to divide the Luminal group into two subgroups (Luminal A and B) [ 138 , 139 ]. The normal breast-like subtype has subsequently been omitted, as it is thought to represent sample contamination by normal mammary glands. In the Cancer Genome Atlas Project (TCGA) over 300 primary tumors were thoroughly profiled (at DNA, RNA, and protein levels) and combined in biological homogenous groups of tumors. The consensus clustering confirmed the distinction of four main breast cancer intrinsic subtypes based on mRNA gene expression levels only (Luminal A, Luminal B, HER2-enriched, and basal-like) [ 140 ]. Additionally, the 5th intrinsic subtype—claudin-low breast cancer was discovered in 2007 in an integrated analysis of human and murine mammary tumors [ 141 ].
In 2009, Parker et al. developed a 50-gene signature for subtype assignment, known as PAM50, that could reliably classify particular breast cancer into the main intrinsic subtypes with 93% accuracy [ 142 ]. PAM50 is now clinically implemented worldwide using the NanoString nCounter ® , which is the basis for the Prosigna ® test. The Prosigna ® combines the PAM50 assay as well as clinical information to assess the risk of distant relapse estimation in postmenopausal women with hormone receptor-positive, node-negative, or node-positive early-stage breast cancer patients, and is a daily-used tool assessing the indication of adjuvant chemotherapy [ 143 , 144 , 145 ].
4.2. Luminal Breast Cancer
Luminal breast cancers are ER-positive tumors that comprise almost 70% of all cases of breast cancers in Western populations [ 146 ]. Most commonly Luminal-like cancers present as IBC of no special subtype, but they may infrequently differentiate into invasive lobular, tubular, invasive cribriform, mucinous, and invasive micropapillary carcinomas [ 147 , 148 ]. Two main biological processes: proliferation-related pathways and luminal-regulated pathways distinguish Luminal-like tumors into Luminal A and B subtypes with different clinical outcomes.
Luminal A tumors are characterized by presence of estrogen-receptor (ER) and/or progesterone-receptor (PR) and absence of HER2. In this subtype the ER transcription factors activate genes, the expression of which is characteristic for luminal epithelium lining the mammary ducts [ 149 , 150 ]. It also presents a low expression of genes related to cell proliferation [ 151 ]. Clinically they are low-grade, slow-growing, and tend to have the best prognosis.
In contrast to subtype A, Luminal B tumors are higher grade and has worse prognosis. They are ER positive and may be PR negative and/or HER2 positive. Additionally, it has high expression of proliferation-related genes (e.g., MKI67 and AURKA) [ 152 , 153 , 154 ]. This subtype has lower expression of genes or proteins typical for luminal epithelium such as the PR [ 150 , 155 ] and FOXA1 [ 146 , 156 ], but not the ER [ 157 ]. ER is similarly expressed in both A and B subtypes and is used to distinguish luminal from non-luminal disease.
4.3. HER2-Enriched Breast Cancer
The HER2-enriched group makes up 10–15% of breast cancers. It is characterized by the high expression of the HER2 with the absence of ER and PR. This subtype mainly expresses proliferation—related genes and proteins (e.g., ERBB2/HER2 and GRB7), rather than luminal and basal gene and protein clusters [ 154 , 156 , 157 ]. Additionally, in the HER2-enriched subtype there is evidence of mutagenesis mediated by APOBEC3B. APOBEC3B is a subclass of APOBEC cytidine deaminases, which induce cytosine mutation biases and is a source of mutation clusters [ 158 , 159 , 160 ].
HER2-enriched cancers grow faster than luminal cancers and used to have the worst prognosis of subtypes before the introduction of HER2-targeted therapies. Importantly, the HER2-enriched subtype is not synonymous with clinically HER2-positive breast cancer because many ER-positive/HER2-positive tumors qualify for the luminal B group. Moreover, about 30% of HER2-enriched tumors are classified as clinically HER2-negative based on immunohistochemistry (IHC) and/or fluorescence in situ hybridization (FISH) methods [ 161 ].
4.4. Basal-Like/Triple-Negative Breast Cancer
The Triple-Negative Breast Cancer (TNBC) is a heterogeneous collection of breast cancers characterized as ER-negative, PR-negative, and HER2-negative. They constitute about 20% of all breast cancers. TNBC is more common among women younger than 40 years of age and African-American women [ 161 ]. The majority (approximately 80%) of breast cancers arising in BRCA1 germline mutation are TNBC, while 11–16% of all TNBC harbor BRCA1 or BRCA2 germline mutations. TNBC tends to be biologically aggressive and is often associated with a worse prognosis [ 162 ]. The most common histology seen in TNBC is infiltrating ductal carcinoma, but it may also present as medullary-like cancers with a prominent lymphocytic infiltrate; metaplastic cancers, which may show squamous or spindle cell differentiation; and rare special type cancers like adenoid cystic carcinoma (AdCC) [ 163 , 164 , 165 ].
The terms basal-like and TNBC have been used interchangeably; however, not all TNBC are of the basal type. On gene expression profiling, TNBCs can be subdivided into six subtypes: basal-like (BL1 and BL2), mesenchymal (M), mesenchymal stem-like (MSL), immunomodulatory (IM), and luminal androgen receptor (LAR), as well as an unspecified group (UNS) [ 166 , 167 ]. However, the clinical relevance of the subtyping still unclear, and more research is needed to clarify its impact on TNBC treatment decisions [ 168 ].
4.5. Claudin-Low Breast Cancer
Claudin-low (CL) breast cancers are poor prognosis tumors being mostly ER-negative, PR-negative, and HER2-negative. CL tumors account for 7–14% of all invasive breast cancers [ 147 ]. No differences in survival rates were observed between claudin-low tumors and other poor-prognosis subtypes (Luminal B, HER2-enriched, and Basal-like). CL subtype is characterized by the low expression of genes involved in cell-cell adhesion, including claudins 3, 4, and 7, occludin, and E-cadherin. Besides, these tumors show high expression of epithelial-mesenchymal transition (EMT) genes and stem cell-like gene expression patterns [ 169 , 170 ]. Moreover, CL tumors have marked immune and stromal cell infiltration [ 171 ]. Due to their less differentiated state and a preventive effect of the EMT-related transcription factor, ZEB1 CL tumors are often genomically stable [ 172 , 173 ].
4.6. Surrogate Markers Classification
In clinical practice, the key question is the discrimination between patients who will or will not benefit from particular therapies. By using molecular assays, more patients can be spared adjuvant chemotherapy, but these tests are associated with significant costs. Therefore, surrogate subgroups based on pathological morphology and widely available immunohistochemical (IHC) markers are used as a tool for risk stratification and guidance of adjuvant therapy [ 174 ]. A combination of the routine pathological markers ER, PR, and HER2 is used to classify tumors into intrinsic subtypes [ 175 ]. Semiquantitative evaluation of Ki-67 and PR is helpful for further typing of the Luminal subtype [ 176 , 177 ]. Moreover, evaluation of cytokeratin 5/6 and epidermal growth factor receptor is utilized to identify the Basal-like breast cancer among the TNBC [ 178 ].
In St. Gallen’s 2013 guidelines the IHC-based surrogate subtype classification was recommended for clinical decision making [ 179 ]. However, these IHC-based markers are only a surrogate and cannot establish the intrinsic subtype of any given cancer, with discordance rates between IHC-based markers and gene-based assays as high as 30% [ 180 ].
4.7. American Joint Committee on Cancer Classification
The baseline tool to estimate the likely prognosis of patients with breast cancer is the AJCC staging system that includes grading, immunohistochemistry biomarkers, and anatomical advancement of the disease. Since its inception in 1977, the American Joint Committee on Cancer (AJCC) has published an internationally accepted staging system based on anatomic findings: tumor size (T), nodal status (N), and metastases (M). However, gene expression profiling has identified several molecular subtypes of breast cancer [ 181 ]. The eighth edition of the AJCC staging manual (2018), outlines a new prognostic staging system for breast cancer that, in addition to anatomical features, acknowledges biological factors [ 182 ]. These factors—ER, PR, HER2, grade, and multigene assays—are recommended in practice to define prognosis [ 183 , 184 ].
The most widely used histologic grading system of breast cancer is the Elston-Ellis modification [ 185 ] of Scarff-Bloom-Richardson grading system [ 186 ], also known as the Nottingham grading system. The grade of a tumor is determined by assessing morphologic features: (a) formation of tubules, (b) mitotic count, (c) variability, and the size and shape of cellular nuclei. A score between 1 (most favorable) and 3 (least favorable) is assigned for each feature. Grade 1 corresponds to combined scores between 3 and 5, grade 2 corresponds to a combined score of 6 or 7, and grade 3 corresponds to a combined score of 8 or 9.
In addition to grading and biomarkers, the commercially available multigene assays provide additional prognostic information suitable for incorporation in the AJCC 8th edition. The 21-gene assay Oncotype DX ® assessed by reverse transcription-polymerase chain reaction (RT-PCR) was the only assay sufficiently evaluated and included in the staging system. This assay is valuable in the staging of patients with hormone receptor-positive, HER2-negative, node-negative tumors that are <5 cm. Patients with results of the assay (Recurrence Score) less than 11 had excellent disease-free survival at 6.9 years of 98.6% with endocrine therapy alone [ 187 ]. Hence, adjuvant systemic chemotherapy can be safely omitted in patients with a low-risk multigene assay [ 188 ].
The AJCC staging manual includes a pathological and a clinical-stage group. The clinical prognostic stage group should be utilized in all patients on initial evaluation before any systemic therapy. Clinical staging uses the TNM anatomical information, grading, and expression of these three biomarkers. When patients undergo surgical resection of their primary tumor, the post-resection anatomic information coupled with the pretreatment biomarker findings results in the final Pathologic Prognostic Stage Group.
The recent update of breast cancer staging by the biologic markers improved the outcome prediction in comparison to prior staging based only on anatomical features of the disease. The validation studies involving the reassessment of the Surveillance, Epidemiology, and End Results (SEER) database ( n = 209,304, 2010–2014) and the University of Texas MD Anderson Cancer Center database ( n = 3327, years of treatment 2007–2013) according to 8th edition AJCC manual proved the more accurate prognostic information [ 189 , 190 ].
5. Prognostic Biomarkers
5.1. estrogen receptor.
Estrogen receptor (ER) is an important diagnostic determinant since approximately 70–75% of invasive breast carcinomas are characterized by significantly enhanced ER expression [ 191 , 192 ]. Current practice requires the measurement of ER expression on both—primary invasive tumors and recurrent lesions. This procedure is mandatory to provide the selection of those patients who will most benefit from the implementation of the endocrine therapy mainly selective estrogen receptor modulators, pure estrogen receptor downregulators, or third-generation aromatase inhibitors [ 193 ]. Even though the diagnosis of altered expression of ER is particularly relevant in terms of the proper therapy selection, ER expression might also constitute a predictive factor—patients with high ER expression usually present significantly better clinical outcomes [ 194 ]. A relationship was observed between ER expression and the family history of breast cancer which further facilitates the utility of ER expression as a diagnostic biomarker of breast cancer especially in cases of familial risk [ 195 ]. Besides, Konan et al. reported that ERα-36 expression could constitute one of the potential targets of PR-positive cancers and a prognostic marker at the same time [ 196 ].
5.2. Progesterone Receptor
PR is highly expressed (>50%) in patients with ER-positive while quite rarely in those with ER-negative breast cancer [ 197 ]. PR expression is regulated by ER therefore, physiological values of PR inform about the functional ER pathway [ 197 ]. However, both ER and PR are abundantly expressed in breast cancer cells and both are considered as diagnostic and prognostic biomarkers of breast cancer (especially ER-positive ones) [ 198 ]. Greater PR expression is positively associated with the overall survival, time to recurrence, and time to either treatment failure or progression while lowered PR levels are usually related to a more aggressive course of the disease as well as poorer recurrence and prognosis [ 199 ]. Thus, favorable management of breast cancer patients highly depends on the assessment of PR expression. Nevertheless, the predictive value of PR expression still remains controversial [ 200 ].
5.3. Human Epidermal Growth Factor Receptor 2
The expression of human epidermal growth factor receptor 2 (HER2) accounts for approximately 15–25% of breast cancers and its status is primarily relevant in the choice of proper management with breast cancer patients; HER2 overexpression is one of the earliest events during breast carcinogenesis [ 201 ]. Besides, HER2 increases the detection rate of metastatic or recurrent breast cancers from 50% to even more than 80% [ 202 ]. Serum HER2 levels are considered to be a promising real-time marker of tumor presence or recurrence [ 203 ]. HER2 amplification leads to further overactivation of the pro-oncogenic signaling pathways leading to uncontrolled growth of cancer cells which corresponds with poorer clinical outcomes in the case of HER2-positive cancers [ 204 ]. Overexpression of HER2 also correlates with a significantly shorter disease-free period [ 205 ] as well as histologic type, pathologic state of cancer, and a number of axillary nodes with metastatic cancerous cells [ 205 ].
5.4. Antigen Ki-67
The Ki-67 protein is a cellular marker of proliferation and the Ki-67 proliferation index is an excellent marker to provide information about the proliferation of cancerous cells particularly in the case of breast cancer. The proliferative activities determined by Ki-67 reflect the aggressiveness of cancer along with the response to treatment and recurrence time [ 206 ]. Thus, Ki-67 is crucial in terms of the choice of the proper treatment therapy and the potential follow-ups due to recurrence. Though, due to several limitations of the analytical validity of Ki-67 immunohistochemistry, Ki-67 expression levels should be considered benevolently in terms of definite treatment decisions. Ki-67 might be considered as a potential prognostic factor as well; according to a meta-analysis of 68 studies involving 12,155 patients, the overexpression of Ki-67 is associated with poorer clinical outcomes of patients [ 207 ]. High expression of Ki-67 also reflects poorer survival rates of breast cancer patients [ 208 ]. There are speculations whether Ki-67 could be considered as a potential predictive marker, however, such data is still limited and contradictory.
Mib1 (antibody against Ki-67) proliferation index remains a reliable diagnostic biomarker of breast cancer, similarly to Ki-67. A decrease in both Mib1 and Ki-67 expression levels is associated with a good response of breast cancer patients to preoperative treatment [ 209 ]. Mib1 levels are significantly greater in patients with concomitant p53 mutations [ 210 ]. Mib1 assessment might be especially useful in cases of biopsy specimens small in size, inappropriate for neither mitotic index nor S-phase fraction evaluation [ 211 ].
5.6. E-Cadherin
E-cadherin is a critical protein in the epithelial-mesenchymal transition (EMT); loss of its expression leads to the gradual transformation into mesenchymal phenotype which is further associated with increased risk of metastasis. The utility of E-cadherin as a breast biomarker is yet questionable, however, some research indicated that its expression is potentially associated with several breast cancer characteristics such as tumor size, TNM stage, or lymph node status [ 212 ]. Low or even total loss of E-cadherin expression might be potentially useful in the determination of histologic subtype of breast cancer [ 213 , 214 ]. E-cadherin levels do not seem to be promising in terms of patients’ survival rates assessment, however, there are some reports indicating that higher levels of E-cadherin were associated with shorter survival rates in patients with invasive breast carcinoma [ 213 , 215 ]. Lowered E-cadherin expression is positively associated with lymph node metastasis [ 216 ].
5.7. Circulating Circular RNA
Circulating circular RNAs (circRNAs) belong to the group of non-coding RNA and were quite recently shown to be crucial in terms of several hallmarks of breast carcinogenesis including apoptosis, enhanced proliferation, or increased metastatic potential [ 217 ]. One of the most comprehensively described circRNAs, mostly specific to breast cancer include circFBXW7—which was proposed as a potential diagnostic biomarker as well as therapeutic tool for patients with triple-negative breast cancer (TNBC), as well as hsa_circ_0072309 which is abundantly expressed in breast cancer patients and usually associated with poorer survival rates [ 218 ]. Has_circ_0001785 is considered to be promising as a diagnostic biomarker of breast cancer [ 219 ]. The number of circRNAs dysregulated during breast carcinogenesis is significant; their expression might be either upregulated (e.g., has_circ_103110, circDENND4C) or downregulated (e.g., has_circ_006054, circ-Foxo3) [ 220 ]. Besides, specific circRNAs have been reported in different types of breast cancer such as TNBC, HER2-positive, and ER-positive [ 221 ]. Recently it was showed that an interaction between circRNAs and micro-RNA—namely in the form of Cx43/has_circ_0077755/miR-182 post-transcriptional axis, might predict breast cancer initiation as well as further prognosis. Cx43 is transmembrane protein responsible for epithelial homeostasis that mediates junction intercellular communication and its loss dysregulates post-transcriptional axes in breast cancer initiation [ 222 ].
Loss-of-function mutations in the TP53 (P53) gene have been found in numerous cancer types including osteosarcomas, leukemia, brain tumors, adrenocortical carcinomas, and breast cancers [ 223 , 224 ]. P53 protein is essential for normal cellular homeostasis and genome maintenance by mediating cellular stress responses including cell cycle arrest, apoptosis, DNA repair, and cellular senescence [ 225 ]. The silencing mutation of the P53 gene is evident at an early stage of cancer progression. In breast cancer, the prevalence of TP53 mutations is present in approximately 80% of patients with the TNBC and 10% of patients with Luminal A disease [ 226 ].
There have been many studies showing the prognostic role of p53 loss-of-function mutation in breast cancer [ 227 , 228 ]. However, the missense mutations may alters p53 properties causing not only a loss of wild-type function, but also acquisition novel activities-gain of function [ 229 ]. The IHC status of p53 has been proposed as a specific prognostic factor in TNBC, and a feature that divides TNBC into 2 distinct subgroups: a p53-negative normal breast-like TN subgroup, and a p53-positive basal-like subgroup with worse overall survival [ 230 , 231 , 232 ]. However, there is not enough evidence to utilize p53 gene mutational status or immunohistochemically measured protein for determining standardized prognosis in patients with breast cancer [ 233 ].
5.9. MicroRNA
MicroRNAs (miRNA) are a major class of endogenous non-coding RNA molecules (19–25 nucleotides) that have regulatory roles in multiple pathways [ 234 ]. Some miRNAs are related to the development, progression, and response of the tumor to therapy [ 235 ]. Several studies have investigated abnormally expressed miRNAs as biomarkers in breast cancer tissue samples. According to meta-analysis by Adhami et al. two miRNAs (miRNA-21 and miRNA-210) were upregulated consistently and six miRNAs (miRNA-145, miRNA-139-5p, miRNA-195, miRNA-99a, miRNA-497, and miRNA-205) were downregulated consistently in at least three studies [ 236 ].
The miRNA-21 overexpression was observed in TNBC tissues and was associated with enhanced invasion and proliferation of TNBC cells as well as downregulation of the PTEN expression [ 237 ]. Similarly, the high expression of miRNA-210 is related to tumor proliferation, invasion, and poor survival rates in breast cancer patients [ 238 , 239 ].
The miRNA-145 is an anti-cancer agent having the property of inhibiting migration and proliferation of breast cancer cells via regulating the TGF-β1 expression [ 240 ]. However, the miRNA-145 is downregulated in both plasma and tumors of breast cancer patients [ 241 ]. Similarly, miRNA-139-5p and miRNA-195 have tumor suppressor activity in various cancers [ 242 , 243 ].
Nevertheless, further clinical researches focusing on these miRNAs are needed to utilize them as reproducible, disease-specific markers that have a high level of specificity and sensitivity.
5.10. Tumor-Associated Macrophages
Macrophages are known for their immunomodulatory effects and they can be divided according to their phenotypes into M1- or M2-like states [ 244 , 245 ]. M1 macrophages secrete IL-12 and tumor necrosis factor with antimicrobial and antitumor effects. M2 macrophages produce cytokines, including IL-10, IL-1 receptor antagonist type II, and IL-1 decoy receptor. Therefore, macrophages with M1-like phenotype have been linked to good disease course while M2-like phenotype has been associated with adverse outcome, potentially through immunosuppression and the promotion of angiogenesis and tumor cell proliferation and invasion [ 246 , 247 ]. In literature, tumor-associated macrophages (TAMs) are associated with M2 macrophages which promote tumor growth and metastasis.
For breast cancer, studies have shown that the density of TAMs is related to hormone receptor status, stage, histologic grade, lymph node metastasis, and vascular invasion [ 248 , 249 , 250 , 251 ]. According to meta-analysis conducted by Zhao et al. high density of TAMs was related to overall survival disease-free survival [ 252 ].
Conversely, M1 polarized macrophages are linked to favorable prognoses in various cancers [ 253 , 254 , 255 ]. In breast cancer, the high density of M1-like macrophages predicted improved survival in patients with HER2+ phenotype and may be a potential prognostic marker [ 256 ].
However, further studies are needed to clarify the influence of macrophages on breast cancer biology as well as investigate the role of their intratumoral distribution and surface marker selection.
5.11. Inflammation-Based Models
The host inflammatory and immune responses in the tumor and its microenvironment are critical components in cancer development and progression [ 257 ]. The tumor-induced systemic inflammatory response leads to alterations of peripheral blood white blood cells [ 258 ]. Therefore, the relationship between peripheral blood inflammatory cells may serve as an accessible and early method of predicting patient prognosis. Recent studies have reported the predictive role of the inflammatory cell ratios: neutrophil-to-lymphocyte ratio, the lymphocyte-to-monocyte ratio, and the platelet-to-lymphocyte ratio for prognosis in different cancers [ 258 , 259 , 260 , 261 ].

5.11.1. The Neutrophil-to-Lymphocyte Ratio (NLR)
In an extensive study on 27,031 cancer patients, Proctor et al. analyzed the prognostic value of NLR and found a significant relationship between NLR and survival in various cancers including breast cancer [ 262 ]. There are pieces of evidence of the role of lymphocytes in breast cancer immunosurveillance [ 263 , 264 ]. Opposingly neutrophils suppress the cytolytic activity of lymphocytes, leading to enhanced angiogenesis and tumor growth and progression [ 265 ].
Azab et al. first reported that NLR before chemotherapy was an independent factor for long-term mortality and related it to age and tumor size in breast cancer [ 266 ]. In a recent meta-analysis by Guo et al., performed on 17,079 individuals, the high NLR level was associated with both poor overall survival as well as disease-free survival for breast cancer patients. Moreover, it was reported that association between NLR and overall survival was stronger in TNBC patients than in HER2-positive ones [ 267 ].
5.11.2. Lymphocyte-to-Monocyte Ratio
The association of the lymphocyte-to-monocyte ratio (LMR) with patients’ prognosis has been reported for several cancers [ 268 , 269 ]. As lymphocytes have an antitumor activity by inducing cytotoxic cell death and inhibiting tumor proliferation [ 270 ], the monocytes are involved in tumorigenesis, including differentiation into TAMs [ 246 , 247 , 271 ]. In the tumor microenvironment, cytokines, and free radicals that are secreted by monocytes and macrophages are associated with angiogenesis, tumor cell invasion, and metastasis [ 271 ].
A meta-analysis investigating the prognostic effect of LMR showed that low LMR levels are associated with shorter overall survival outcomes in Asian populations, TNBC patients, and patients with non-metastatic and mixed stages [ 272 ]. Moreover, high LMR levels are associated with favorable disease-free survival of breast cancer patients under neoadjuvant chemotherapy [ 273 ].
5.11.3. Platelet-to-Lymphocyte Ratio (PLR)
A high platelet count has been associated with poor prognosis in several types of cancers [ 274 , 275 , 276 ]. Platelets contain both pro-inflammatory molecules and cytokines (P-selectin, CD40L, and interleukin (IL)-1, IL-3, and IL-6) and many anti-inflammatory cytokines. Tumor angiogenesis and growth may be stimulated by the secretion of platelet-derived growth factor, vascular endothelial growth factor, transforming growth factor-beta, and platelet factor 4 [ 277 , 278 , 279 ].
A meta-analysis study investigated the prognostic importance of PLR by analyzing 5542 breast cancer patients. High PLR level was associated with poor prognosis (overall survival and disease-free survival), yet, its prognostic value was not determined for molecular subtypes of breast cancer. Nevertheless, an association was found between PLR and clinicopathological features of the tumor, including stage, lymph node metastasis, and distant metastasis [ 280 ]. In the aforementioned meta-analysis, there was a difference in the incidence of high levels of PLR between HER2 statuses [ 280 ], while other studies found a difference between hormone ER or PR statuses [ 281 , 282 ].
6. Treatment Strategies
6.1. surgery.
There are two major types of surgical procedures enabling the removal of breast cancerous tissues and those include (1) breast-conserving surgery (BCS) and (2) mastectomy. BCS—also called partial/segmental mastectomy, lumpectomy, wide local excision, or quadrantectomy—enables the removal of the cancerous tissue with simultaneous preservation of intact breast tissue often combined with plastic surgery technics called oncoplasty. Mastectomy is a complete removal of the breast and is often associated with immediately breast reconstruction. The removal of affected lymph nodes involves sentinel lymph node biopsy (SLNB) and axillary lymph node dissection (ALND). Even though BCS seems to be highly more beneficial for patients, those who were treated with this technique often show a tendency for a further need for a complete mastectomy [ 283 ]. However, usage of BCS is mostly related to significantly better cosmetic outcomes, lowered psychological burden of a patient, as well as reduced number of postoperative complications [ 284 ]. Guidelines of the European Society for Medical Oncology (ESMO) for patients with early breast cancer make the choice of therapy dependent to tumor size, feasibility of surgery, clinical phenotype, and patient’s willingness to preserve the breast [ 285 ].
6.2. Chemotherapy
Chemotherapy is a systemic treatment of BC and might be either neoadjuvant or adjuvant. Choosing the most appropriate one is individualized according to the characteristics of the breast tumor; chemotherapy might also be used in the secondary breast cancer. Neoadjuvant chemotherapy is used for locally advanced BC, inflammatory breast cancers, for downstaging large tumors to allow BCS or in small tumors with worse prognostics molecular subtypes (HER2 or TNBC) which can help to identify prognostics and predictive factors of response and can be provided intravenously or orally. Currently, treatment includes a simultaneous application of schemes 2–3 of the following drugs—carboplatin, cyclophosphamide, 5-fluorouracil/capecitabine, taxanes (paclitaxel, docetaxel), and anthracyclines (doxorubicin, epirubicin). The choice of the proper drug is of major importance since different molecular breast cancer subtypes respond differently to preoperative chemotherapy [ 286 ]. Preoperative chemotherapy is comparably effective to postoperative chemotherapy [ 287 ].
Even though chemotherapy is considered to be effective, its usage very often leads to several side effects including hair loss, nausea/vomiting, diarrhea, mouth sores, fatigue, increased susceptibility to infections, bone marrow supression, combined with leucopenia, anaemia, easier bruising or bleeding; other less frequent side effects include cardiomyopathy, neuropathy, hand-foot syndrome, impaired mental functions. In younger women, disruptions of the menstrual cycle and fertility issues might also appear. Special form of chemotherapy is electrochemotherapy which can be used in patients with breast cancer that has spread to the skin, however, it is still quite uncommon and not available in most clinics.
6.3. Radiation Therapy
Radiotherapy is local treatment of BC, typically provided after surgery and/or chemotherapy. It is performed to ensure that all of the cancerous cells remain destroyed, minimizing the possibility of breast cancer recurrence. Further, radiation therapy is favorable in the case of metastatic or unresectable breast cancer [ 288 ]. Choice of the type of radiation therapy depends on previous type of surgery or specific clinical situation; most common techniques include breast radiotherapy (always applied after BC), chest-wall radiotherapy (usually after mastectomy), and ‘breast boost’ (a boost of high-dose radiotherapy to the place of tumor bed as a complement of breast radiotherapy after BCS). Regarding breast radiotherapy specifically, several types are distinguished including
- (1) intraoperative radiation therapy (IORT)
- (2) 3D-conformal radiotherapy (3D-CRT)
- (3) intensity-modulated radiotherapy (IMRT)
- (4) brachytherapy—which refers to internal radiation in contrast to other above-mentioned techniques.
Irritation and darkening of the skin exposed to radiation, fatigue, and lymphoedema are one of the most common side effects of radiation therapy applied in breast cancer patients. Nonetheless, radiation therapy is significantly associated with the improvement of the overall survival rates of patients and lowered risk of recurrence [ 289 ].
6.4. Endocrinal (Hormonal) Therapy
Endocrinal therapy might be used either as a neoadjuvant or adjuvant therapy in patients with Luminal–molecular subtype of BC; it is effective in cases of breast cancer recurrence or metastasis. Since the expression of ERs, a very frequent phenomenon in breast cancer patients, its blockage via hormonal therapy is commonly used as one of the potential treatment modalities. Endocrinal therapy aims to lower the estrogen levels or prevents breast cancer cells to be stimulated by estrogen. Drugs that block ERs include selective estrogen receptor modulators (SERMs) (tamoxifen, toremifene) and selective estrogen receptor degraders (SERDs) (fulvestrant) while treatments that aim to lower the estrogen levels include aromatase inhibitors (AIs) (letrozole, anastrazole, exemestane) [ 290 , 291 ]. In the case of pre-menopausal women, ovarian suppression induced by oophorectomy, luteinizing hormone-releasing hormone analogs, or several chemotherapy drugs, are also effective in lowering estrogen levels [ 292 ]. However, approximately 50% of hormonoreceptor-positive breast cancer become progressively resistant to hormonal therapy during such treatment [ 293 ]. Endocrinal therapy combined with chemotherapy is associated with the reduction of mortality rates amongst breast cancer patients [ 294 ].
6.5. Biological Therapy
Biological therapy (targeted therapy) can be provided at every stage of breast therapy– before surgery as neoadjuvant therapy or after surgery as adjuvant therapy. Biological therapy is quite common in HER2-positive breast cancer patients; major drugs include trastuzumab, pertuzumab, trastuzumab deruxtecan, lapatinib, and neratinib [ 295 , 296 , 297 , 298 , 299 ]. Further, the efficacy of angiogenesis inhibitors such as a recombinant humanized monoclonal anti-VEGF antibody (rhuMAb VEGF) or bevacizumab are continuously investigated [ 300 ].
In the case of Luminal, HER2-negative breast cancer, pre-menopausal women more often receive everolimus -TOR inhibitor with exemestane while postmenopausal women often receive CDK 4–6 inhibitor palbociclib or ribociclib simultaneously, combined with hormonal therapy [ 301 , 302 , 303 ]. Two penultimate drugs along with abemaciclib and everolimus can also be used in HER2-negative and estrogen-positive breast cancer [ 304 , 305 ]. Atezolizumab is approved in triple-negative breast cancer, while denosumab is approved in case of metastasis to the bones [ 306 , 307 , 308 ].
7. Conclusions
In this review, we aimed to summarize and update the current knowledge about breast cancer with an emphasis on its current epidemiology, risk factors, classification, prognostic biomarkers, and available treatment strategies. Since both the morbidity and mortality rates of breast cancer have significantly increased over the past decades, it is an urgent need to provide the most effective prevention taking into account that modifiable risk factors might be crucial in providing the reduction of breast cancer incidents. So far, mammography and sonography is the most common screening test enabling quite an early detection of breast cancer. The continuous search for prognostic biomarkers and targets for the potential biological therapies has significantly contributed to the improvement of management and clinical outcomes of breast cancer patients.
Author Contributions
Conceptualization, A.F., R.S. and A.S.; critical review of literature, S.Ł., M.C., A.F., J.B., R.S., A.S.; writing—original draft preparation, M.C., A.F.; writing—review and editing, S.Ł., M.C., A.F., J.B., R.S., A.S.; supervision, R.S. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Your Account
Manage your account, subscriptions and profile.
MyKomen Health
ShareForCures
In Your Community
In Your Community
View resources and events in your local community.
Change your location:

Susan G. Komen®
One moment can change everything.
What’s New in Breast Cancer
This section gives an overview of new breast cancer treatment breakthroughs and recent developments in research that are fueling new ways to assess risk, and prevent, detect, diagnose and treat breast cancer. Advances in breast cancer care are evaluated through a rigorous process that includes clinical trials and regulatory approvals before being considered standards of care and included in breast cancer care guidelines. Komen’s research team monitors the rapidly evolving breast cancer landscape, and here we will highlight new breast cancer treatment breakthroughs, innovations in technology or key advances that may be added or are new to guidelines. We will share these research advancements to empower patients with knowledge to help them make informed decisions with their doctors.
Use these links to jump to the topics below.
- Emerging Areas in Metastatic Breast Cancer Treatment
- Clinical Trials
Treatments and Drugs
For patients, new treatments can mean more options and more hope. Researchers are working to develop new breast cancer treatment breakthroughs, such as more effective drugs that will specifically target breast cancer cells, minimize side effects and prevent breast cancer cells from coming back. While some treatments increase the effectiveness of existing drugs, others may offer new, innovative strategies for attacking tumor cells.
As of August 2023, the following new treatments and drugs are currently in clinical trials and have not yet received FDA approval:
- A new antibody-drug conjugate called datopotamab deruxtecan (Dato-DXd) is currently being evaluated in three Phase 3 clinical trials for advanced estrogen receptor-positive (ER+) [2] breast cancer, metastatic triple negative [ 3 ] breast cancer and early triple negative [ 4 ] breast cancer (TNBC). Dato-DXd specifically targets a protein called TROP2, a biomarker that can be used to target cancer cells instead of healthy cells. Another TROP2-targeting therapy called sacituzumab govitecan has already been approved for TNBC and estrogen-receptor-positive breast cancer. Dato-DXd uses a different chemotherapy drug and delivery system compared to sacituzumab govitecan.
- HER2 is a common treatment target for breast cancer. This new drug targets HER3, a biomarker related to HER2, which is associated with poor breast cancer outcomes. About 10% to 20% of newly diagnosed breast cancers are HER2-positive. At the 2023 American Society for Clinical Oncology (ASCO) Annual Meeting, researchers announced positive results for a Phase 2 clinical trial studying HER3-DXd, a new HER3-targeting antibody-drug conjugate for people with metastatic breast cancer . [ 1 ]. While the study found that 35% of patients responded positively to HER3-DXd, researchers will continue to evaluate which patients could benefit most from this drug through future Phase 3 clinical trials.
- CDK4/6 inhibitors are commonly used to treat estrogen receptor-positive breast cancer, but a new CDK4/6 inhibitor called trilaciclib is being tested to treat TNBC. Results from a Phase 2 clinical trial showed that trilaciclib improved outcomes for people with advanced TNBC, and the drug is currently being evaluated in the Phase 3 PRESERVE 2 clinical trial [ 5 ]. Researchers believe that unlike currently available CDK4/6 inhibitors, trilaciclib may improve response to immunotherapy and mitigate some of the side effects of chemotherapy . If this clinical trial is successful, this would be the first CDK4/6 inhibitor approved for people with TNBC.
New and improved technologies may be able to increase the speed and accuracy of detecting, diagnosing or monitoring breast cancer for progression and response to treatment.
- Doctors may use PET scans, or positron emission tomography, to scan for evidence that breast cancer has spread or metastasized. Once breast cancer has spread, the metastases may have evolved to a different type of breast cancer than the original tumor. These differences mean the metastases and the original tumor may not respond to the same treatments. A diagnostic imaging agent called Cerianna (fluoroestradiol F-18 or FES PET) allows doctors to use PET scans to learn if estrogen receptors are present in metastatic lesions. If a person has metastatic lesions that are estrogen receptor-positive, they may respond well to hormone therapy. This agent was recently incorporated in the National Comprehensive Cancer Network (NCCN) guidelines [ 6 ] as an option for some people with metastatic or recurrent estrogen receptor-positive breast cancer to consider [ 7 ].
- Ovarian suppression increases the effectiveness of hormone therapy in some premenopausal women but comes with additional side effects that can affect quality of life. A study presented at the 2022 San Antonio Breast Cancer Symposium [ 8 ] suggests that the Breast Cancer Index , a tumor profiling test that looks at genes to predict how likely a cancer is to metastasize, may be able to identify premenopausal women that would benefit most from ovarian suppression. This test would give doctors a new tool to personalize treatment for premenopausal women with estrogen receptor-positive breast cancer. More data are needed to confirm these results.
- Doctors are getting closer to identifying which patients with early HER2-positive breast cancer can safely avoid chemotherapy by using the HER2DX genomic test. HER2DX is the first test specifically designed to identify HER2-positive patients at high and low risk for recurrence . For some people, being able to avoid chemotherapy without comprising long-term outcomes will lead to a better quality of life.

Research can take decades to reach the bedside, but what discoveries are just around the corner for patients? Susan G. Komen shares all of this and more through Breast Cancer Breakthroughs, a virtual education series focusing on the new science and technology advancements that are poised to make a difference for patients in the near future. Sign up for Breast Cancer Breakthroughs to never miss an episode.

Kimberly’s Story: Finding Joy in the Midst of a Metastatic Breast Cancer Diagnosis
After Kimberly Reinika’s mother passed away in 2019 from ovarian cancer, she worried that it would ultimately take her life, too. “That was the cancer I was checking for,” she said.
Approaches to Care
With knowledge gained from clinical trials, researchers are seeking new ways to improve patient outcomes while using existing drugs. Some new breast cancer treatment breakthroughs are the result of combining certain drugs, finding which patients can skip certain elements of treatment or changing the order of their treatments to maximize effectiveness or minimize side effects.
- Patients with early estrogen receptor-positive breast cancer generally have a good prognosis, but some people have a higher risk of recurrence for as long as 20 years. Researchers are seeking new strategies to reduce this risk of recurrence. CDK4/6 inhibitors are used to treat advanced breast cancer, but the Phase 3 NATALEE clinical trial, presented at the 2023 American Society of Clinical Oncology Annual Meeting [ 9 ], found that using the CDK4/6 inhibitor ribociclib for two years in the adjuvant setting reduced the risk of recurrence for people with estrogen receptor-positive breast cancer.
- Inflammatory breast cancer is difficult to diagnose because its symptoms often mimic infections. Additionally, because some medical professionals don’t see it often, they may lack experience in recognizing and treating inflammatory breast cancer. In partnership with the Inflammatory Breast Cancer Research Foundation and the Milburn Foundation, Susan G. Komen launched a first-of-its kind diagnostic tool for inflammatory breast cancer. Through this scoring system, the tool considers the defining features of inflammatory breast cancer and provides data that can help providers accurately determine whether a person has inflammatory breast cancer. The goal of this tool is to increase the accuracy of diagnosing inflammatory breast cancer so that people will receive the appropriate care they need to treat this aggressive disease.
- Immunotherapy targets the immune system to help the body fight off tumors. Immunotherapy is currently only available for some patients with triple negative breast cancer, but researchers are aiming to bring this cutting edge therapy to more people. In a recent announcement [ 10 ], positive results were announced for a clinical trial that evaluated the immunotherapy drug pembrolizumab in patients with early estrogen receptor-positive breast cancer. Komen will be closely monitoring the results of this study at upcoming scientific conferences and hopes to see more promising data suggesting that a new treatment option may soon be available for patients with early estrogen receptor-positive breast cancer.
- Clinical trials are often designed using the maximum tolerated dose of a drug. However, many drugs may give the same effect with a smaller dose that results in fewer side effects for the patient. The X-7/7 clinical trial, which was presented at the 2023 ASCO Annual Meeting, tested the impact of a new treatment schedule for the chemotherapy drug capecitabine to treat metastatic breast cancer. Researchers found that people who took a higher dose of capecitabine over fewer days had fewer side effects and were able to remain on their treatment longer compared to the standard regimen. This new approach can improve the quality of life for those living with metastatic breast cancer without compromising the effectiveness of their treatments.
Komen will be closely monitoring the results of these studies and more at upcoming scientific conferences and hopes to see more promising data regarding new ways to prevent, detect, diagnose and treat breast cancer.

It Looks Promising: Uncovering New Possibilities in Breast Cancer Prevention
Is breast cancer prevention possible? Komen Scientific Advisory Board Member Dr. Kornelia Polyak is exploring a new strategy to identify and eliminate cell precursors from which tumors can grow.

Help discover cures to breast cancer, faster. New treatment breakthroughs for breast cancer come from researchers learning from people who have breast cancer, but our current data sources only represent a small portion of the breast cancer community. Help us discover the cures to breast cancer, faster, by joining ShareForCures.
What’s New in Breast Cancer References
- Hamilton, E. P., et al. (2023). “A phase 2 study of HER3-DXd in patients (pts) with metastatic breast cancer (MBC).” Journal of Clinical Oncology 41(16_suppl): 1004-1004. https://meetings.asco.org/abstracts-presentations/219699
- https://classic.clinicaltrials.gov/ct2/show/NCT05104866
- https://clinicaltrials.gov/study/NCT05374512
- https://classic.clinicaltrials.gov/ct2/show/NCT05629585
- https://classic.clinicaltrials.gov/ct2/show/NCT04799249
- https://www.gehealthcare.com/about/newsroom/press-releases/ge-healthcare-announces-fes-pet-imaging-recommendation-in-nccn-clinical-practice-guidelines-in-oncology-nccn-guidelines
- https://www.nccn.org/patients/guidelines/content/PDF/breast-invasive-patient.pdf (page 16)
- https://www.sabcs.org/Portals/SABCS2016/2022%20SABCS/SABCS%202022%20Abstract%20Report.pdf?ver=2022-12-08-111637-860
- Stroyakovskiy, D., et al. (2023). “Ribociclib and endocrine therapy as adjuvant treatment in patients with HR+/HER2- early breast cancer: Primary results from the phase III NATALEE trial.” Journal of Clinical Oncology 41(17_suppl): LBA500-LBA500.
- https://www.merck.com/news/merck-announces-phase-3-keynote-756-trial-met-primary-endpoint-of-pathological-complete-response-pcr-rate-in-patients-with-high-risk-early-stage-er-her2-breast-cancer/
TOOLS & RESOURCES
NEED HELP OR MORE INFORMATION?
1-877 GO KOMEN (1-877-465-6636)
Educational Resources
Komen Financial Assistance Program
Breast Cancer Research

Breast Cancer Risk Factors
Breast Cancer Research is presenting our Retrospective Collection on "Breast Cancer Risk Factors." Celebrating 'Breast Cancer Awareness Month (1 October- 31 October)', with this Collection, we aim to gain valuable insights into the multifaceted aspects of breast cancer risk to promote awareness, prevention, and early detection.
NEW CROSS-JOURNAL COLLECTIONS Find out more by clicking the links below:
Artif icial Intelligence in Breast Imaging PDGFB in Br east Cancer Initiation,Progression, and Metastasis

Aims and scope
- Most accessed
Weakly-supervised deep learning models enable HER2-low prediction from H &E stained slides
Authors: Renan Valieris, Luan Martins, Alexandre Defelicibus, Adriana Passos Bueno, Cynthia Aparecida Bueno de Toledo Osorio, Dirce Carraro, Emmanuel Dias-Neto, Rafael A. Rosales, Jose Marcio Barros de Figueiredo and Israel Tojal da Silva
Validation of an AI-based solution for breast cancer risk stratification using routine digital histopathology images
Authors: Abhinav Sharma, Sandy Kang Lövgren, Kajsa Ledesma Eriksson, Yinxi Wang, Stephanie Robertson, Johan Hartman and Mattias Rantalainen
The SEMA3F-NRP1/NRP2 axis is a key factor in the acquisition of invasive traits in in situ breast ductal carcinoma
Authors: Núria Moragas, Patricia Fernandez-Nogueira, Leire Recalde-Percaz, Jamie L. Inman, Anna López-Plana, Helga Bergholtz, Aleix Noguera-Castells, Pedro J. del Burgo, Xieng Chen, Therese Sorlie, Pere Gascón, Paloma Bragado, Mina Bissell, Neus Carbó and Gemma Fuster
Association of Life’s Essential 8 cardiovascular health with breast cancer incidence and mortality according to genetic susceptibility of breast cancer: a prospective cohort study
Authors: Yan Zhao, Yang Song, Xiangmin Li and Ayao Guo
Micrometastases in axillary lymph nodes in breast cancer, post-neoadjuvant systemic therapy
Authors: Janghee Lee, Seho Park, Soong June Bae, Junghwan Ji, Dooreh Kim, Jee Ye Kim, Hyung Seok Park, Sung Gwe Ahn, Seung Il Kim, Byeong-Woo Park and Joon Jeong
Most recent articles RSS
View all articles
Serum thymidine kinase 1 activity as a pharmacodynamic marker of cyclin-dependent kinase 4/6 inhibition in patients with early-stage breast cancer receiving neoadjuvant palbociclib
Authors: Nusayba Bagegni, Shana Thomas, Ning Liu, Jingqin Luo, Jeremy Hoog, Donald W. Northfelt, Matthew P. Goetz, Andres Forero, Mattias Bergqvist, Jakob Karen, Magnus Neumüller, Edward M. Suh, Zhanfang Guo, Kiran Vij, Souzan Sanati, Matthew Ellis…
Choosing the right cell line for breast cancer research
Authors: Deborah L Holliday and Valerie Speirs
Triple-negative breast cancer molecular subtyping and treatment progress
Authors: Li Yin, Jiang-Jie Duan, Xiu-Wu Bian and Shi-cang Yu
Breast asymmetry and predisposition to breast cancer
Authors: Diane Scutt, Gillian A Lancaster and John T Manning
Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer
Authors: Suzanne A Eccles, Eric O Aboagye, Simak Ali, Annie S Anderson, Jo Armes, Fedor Berditchevski, Jeremy P Blaydes, Keith Brennan, Nicola J Brown, Helen E Bryant, Nigel J Bundred, Joy M Burchell, Anna M Campbell, Jason S Carroll, Robert B Clarke, Charlotte E Coles…
Most accessed articles RSS

Editor-in-Chief
Lewis Chodosh , University of Pennsylvania, USA

Trending in the Media
Click here to see the most popular articles published in Breast Cancer Research in the past three months.

BCR's 20th Anniversary
20 years ago Breast Cancer Research published its first articles with BMC. Well-respected in the field, the journal has continually placed in the first quartile of the ‘Oncology’ category of Journal Citation Reports. Over the past decade, Breast Cancer Research (BCR) has also become the highest ranked breast cancer focused title in the field.
Look back at the journal’s milestone achievements and article highlights .

Featured Review - Artificial intelligence in mammographic phenotyping of breast cancer risk: a narrative review
In this review, we provide a useful reference for AI researchers investigating image-based breast cancer risk assessment while indicating key priorities and challenges that, if properly addressed, could accelerate the implementation of AI-assisted risk stratification to future refine and individualize breast cancer screening strategies.
Springer Nature Oncology Portfolio
Discover the range of academic oncology titles at Springer Nature here .

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Research progress on factors affecting the sensitivity of breast cancer to radiotherapy: a narrative review
Affiliations.
- 1 Department of Oncology, Affiliated Anhui Provincial Hospital, Bengbu Medical College, Bengbu, China.
- 2 Department of Oncology, The First Affiliated Hospital of USTC, Division of Life Science and Medicine, University of Science and Technology of China, Hefei, China.
- 3 Department of Oncology, The First Affiliated Hospital of University of Science and Technology of China, Hefei, China.
- 4 Department of General Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei, China.
- 5 Anhui Provincial Key Laboratory of Precision Pharmaceutical Preparations and Clinical Pharmacy, Hefei, China.
- PMID: 39145054
- PMCID: PMC11319974
- DOI: 10.21037/tcr-24-71
Background and objective: Radiation therapy (RT) is one of the important components of comprehensive treatment for breast cancer and has important value in improving the control rate of local areas, reducing the chance of recurrence and metastasis after breast cancer surgery, delaying disease progression, and improving the survival of breast cancer patients. The factors that affect the RT sensitivity of breast cancer are important. The above potential predictors of radiation efficacy can provide patients with a predictive method and therefore have significant value in clinical therapy. In this paper, we have summarised the predictive factors of radiotherapy sensitivity by reviewing recent research on breast cancer and focused on the following areas: tumor immune microenvironment (TIME), cancer stem cells, noncoding RNAs, signal transduction pathways, genes, etc. This review aims to provide theoretical basis and reference for improving the efficacy of radiotherapy and experimental individualized treatment of breast cancer.
Methods: We searched the Web of Science database to identify clinical studies published between 2010 and January 2024 that investigated radiotherapy sensitivity. The main findings of the validated studies were summarised.
Key content and findings: Improving the radiosensitivity of breast cancer is essential in the treatment of breast cancer. The radiosensitivity can be improved by modulating immune cells or immunomodulatory factors in the TIME, modulating signal transduction pathways, and other innovative combination therapy strategies. And we also summarized the predictive markers of breast cancer radiosensitivity.
Conclusions: In this paper, we reviewed the literature and summarized the newest research advances on the radiosensitivity of breast cancer patients. This review paper includes the following six aspects: the immune microenvironment, tumor stem cells, signaling pathways, regulation of gene/protein expression, small molecule drugs, and predictive markers for radiosensitivity.
Keywords: Radiosensitivity; breast cancer; cancer stem cells (CSCs); predictive markers; tumor immune microenvironment (TIME).
2024 Translational Cancer Research. All rights reserved.
PubMed Disclaimer
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-71/coif). The authors have no conflicts of interest to declare.
Different drugs combined with radiotherapy…
Different drugs combined with radiotherapy modulated the TIME. (I) 1400W, a small molecule…
The process of combination therapy…
The process of combination therapy to improve the radiosensitivity in breast cancer. (I)…
The process of combination therapy to improve radiosensitivity by reducing BCSCs or the…
Similar articles
- Identification and validation of single-sample breast cancer radiosensitivity gene expression predictors. Sjöström M, Staaf J, Edén P, Wärnberg F, Bergh J, Malmström P, Fernö M, Niméus E, Fredriksson I. Sjöström M, et al. Breast Cancer Res. 2018 Jul 4;20(1):64. doi: 10.1186/s13058-018-0978-y. Breast Cancer Res. 2018. PMID: 29973242 Free PMC article.
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
- Gene expression profiling for guiding adjuvant chemotherapy decisions in women with early breast cancer: an evidence-based and economic analysis. Medical Advisory Secretariat. Medical Advisory Secretariat. Ont Health Technol Assess Ser. 2010;10(23):1-57. Epub 2010 Dec 1. Ont Health Technol Assess Ser. 2010. PMID: 23074401 Free PMC article.
- Predictive factors in radiotherapy for non-small cell lung cancer: present status. Choi N, Baumann M, Flentjie M, Kellokumpu-Lehtinen P, Senan S, Zamboglou N, Kosmidis P. Choi N, et al. Lung Cancer. 2001 Jan;31(1):43-56. doi: 10.1016/s0169-5002(00)00156-2. Lung Cancer. 2001. PMID: 11162866 Review.
- Improving the efficacy of combined radiotherapy and immunotherapy: focusing on the effects of radiosensitivity. Gao Z, Zhao Q, Xu Y, Wang L. Gao Z, et al. Radiat Oncol. 2023 May 24;18(1):89. doi: 10.1186/s13014-023-02278-5. Radiat Oncol. 2023. PMID: 37226275 Free PMC article. Review.
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG) , Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707-16. 10.1016/S0140-6736(11)61629-2 - DOI - PMC - PubMed
- Zhou ZR, Yang ZZ, Yu XL, et al. Highlights on molecular targets for radiosensitization of breast cancer cells: Current research status and prospects. Cancer Med 2018;7:3110-7. 10.1002/cam4.1588 - DOI - PMC - PubMed
- Zhu L, Zhao Y, Liu T, et al. Inhibition of NADPH Oxidase-ROS Signal using Hyaluronic Acid Nanoparticles for Overcoming Radioresistance in Cancer Therapy. ACS Nano 2022;16:18708-28. 10.1021/acsnano.2c07440 - DOI - PMC - PubMed
- Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 2014;25:1544-50. 10.1093/annonc/mdu112 - DOI - PubMed
- Garvin S, Oda H, Arnesson LG, et al. Tumor cell expression of CD163 is associated to postoperative radiotherapy and poor prognosis in patients with breast cancer treated with breast-conserving surgery. J Cancer Res Clin Oncol 2018;144:1253-63. 10.1007/s00432-018-2646-0 - DOI - PMC - PubMed
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- AME Publishing Company
- PubMed Central
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
Shots - Health News
Weight-loss drugs like wegovy may help stave off some cancers.

Yuki Noguchi
Obesity, Cancer, and GLP1s

GLP-1 drugs, like Wegovy and Ozempic, may not be good only for diabetes and weight-loss. They are also showing promise for preventing some cancers. UCG/Universal Images Group/Getty Images hide caption
Drugs like Ozempic, Wegovy and Zepbound have transformed treatment for obesity and diabetes. Now researchers are excited about their potential impact on other conditions, including addiction and sleep apnea — and even cancer.
Scientists see this class of drugs, called GLP-1 agonists, as a breakthrough because of how they act on the brain to regulate the body’s hormones, slow digestion, and tamp down hunger. And in several recent studies, they show early promise in preventing many common cancers — including breast, colon, liver, and ovarian — known to be driven by obesity and excess weight.
“It's a hopeful story, which is, frankly, what people need,” says Arif Kamal, an oncologist specializing in breast cancer as well as chief patient officer at the American Cancer Society.
Though research on GLP-1 drugs is still in its relative infancy, so far studies fairly consistently show their benefit in staving off certain cancers. One research letter published in JAMA Oncology last year, for example, suggests GLP-1 drugs might reduce the risk of colon cancer , even among people who are not overweight. A more recent analysis in JAMA Network Open suggests GLP-1s provide far more protection against cancer for diabetic patients than insulin treatments.
Another recent study presented at the American Society of Clinical Oncologists meeting in June, showed both bariatric surgery and GLP-1 medications dramatically reduce the risk of the 13 obesity-related cancers . Among those who had bariatric surgery, that risk declined by 22% over 10 years compared to those who received no treatment. But among those taking GLP1 medications, risk dropped by a whopping 39%.
“And I think a 39% risk reduction is one of the most impactful risk reductions we've ever really seen,” says Kamal.
GLP-1 agonist drugs were originally developed to treat diabetes nearly two decades ago. Over the past decade, regulators started approving them as treatments for weight loss – first as liraglutide, sold under the brand Saxenda and, more recently, in the form of semaglutide or tirzepatide, under brands like Wegovy and Zepbound.
When it comes to cancer prevention, scientists are finding the link between obesity in cancer is complex and intertwined; the obesity-related cancers are heavily concentrated among organs involved in digestion and metabolism, like the liver and pancreas, for example, as well as among gynecologic cancers, including breast and uterus. Reproductive organs are highly sensitive to the hormone estrogen, which plays a role in allowing cells to grow rapidly during pregnancy, for example.
But Kamal says there’s also an especially close relationship between estrogen and cancer. “What we do know is that estrogen in particular — and possibly some other hormones, but estrogen for sure — drives the growth of many cancers,” he says. And fat cells increase production of estrogen.
That means women today are increasingly susceptible to cancer. Historically, men faced a much higher risk of developing cancers — in large part because they were more likely to engage in high-risk behaviors like smoking or drinking, Kamal says. But in recent years, the high prevalence of obesity among both men and women is closing that gender gap.
Obesity is also likely the most significant driver behind increasing cancer rates among younger adults , he says, just as tobacco was in generations past.
“Unhealthy weight is the smoking of our generation,” Kamal says.
That’s why indications that GLP-1 drugs may help slash that risk is so significant.
What’s more, that ASCO study suggests that GLP-1 drugs have a notable impact on cancer risk, even when patients don’t lose a lot of weight as a result of taking them. In other words, the medications seem to act on a number of the body’s mechanisms to reduce vulnerabilities to cancer.
“We think the protective effects of GLP-1s are probably multifactorial,” says Cindy Lin, resident physician at Case Western Reserve and co-author of the June ASCO study. “Part of it is weight [loss], but other factors may be contributing as well — better glycemic controls, anti-inflammatory effects.”
More research is necessary and inevitable — especially studies looking at the newer weight-loss formulations of GLP-1 medications, says Benjamin Liu, another resident physician at Case Western and co-author of the ASCO study.
He says he’s encouraged by the data so far. “It's very exciting to have, especially since it's more of a noninvasive strategy compared to bariatric surgery, and a lot more patients will be open to it.”
- cancer prevention
- semaglutide
- Share full article
Advertisement
Supported by
Biden Awards $150 Million in Research Grants as Part of Cancer ‘Moonshot’
President Biden has had a deep personal interest in cancer research since his son Beau died of an aggressive brain cancer in 2015.
President Biden Announces $150 Million in Cancer Research Grants
President biden said eight research centers would receive research awards aimed at pioneering new methods of precision cancer surgery as part of his administration’s cancer “moonshoot” initiative..
As all of you know, cancer surgery is an incredibly challenging procedure. It takes the best surgeons in the world, and it takes its toll on families. As Jill and I — as Jill says, it steals time. It steals away hope. Our family knows the feeling, as many here do. Today, we’re announcing $150 million ARPA-H funding for some of the nation’s cutting-edge cancer research institutions. That includes, right here, Tulane University. [cheers] And we’re moving quickly because we know all families touched by cancers are in a race against time. It’s all part of our goal, of our cancer “moonshot,” to end cancer as we know it. Even cure some cancers. We’re mobilizing the whole of country effort to cut American cancer deaths in half by — within 25 years, and boost support for patients and their families. I’m confident in our capacity to do that.

By Zach Montague
Reporting from New Orleans
Freed from the campaign trail and the grinding pursuit of another term, President Biden traveled to New Orleans on Tuesday to focus on a project close to his heart: the “moonshot” effort to sharply cut cancer deaths in the United States that he carried over from his time as vice president and has become a hallmark of his presidency.
Speaking at Tulane University, Mr. Biden and the first lady, Jill Biden, announced eight research centers, including one at Tulane, that will collectively receive $150 million in research awards aimed at pioneering new methods of precision cancer surgery.
Before addressing a crowd on campus, the president and the first lady met with a team of researchers who demonstrated the technology under development at Tulane. It uses imaging of cells on tumor sites to verify for surgeons that cancer cells have been fully removed and to reduce the need for follow-up surgeries.
Standing in front of a sign reading “curing cancer faster,” Mr. Biden described touring cancer centers in Australia and Ireland, and being frustrated by a lack of international collaboration.
“We don’t want to keep information — we want to share it,” he said.
The awards announced on Tuesday are to be made through the Advanced Research Projects Agency for Health , or ARPA-H, which was founded in 2022 and is aimed at driving biomedical innovation.
The other award recipients were Dartmouth College; Johns Hopkins University; Rice University; the University of California, San Francisco; the University of Illinois Urbana-Champaign; the University of Washington; and Cision Vision in Mountain View, Calif.
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- FROM THE ANALYST'S COUCH
- 13 August 2024
The breast cancer drug market
- Laura Ramos García 0 ,
- Kiran Bountra &
- Rachel M. Webster 2
Clarivate, Barcelona, Spain.
You can also search for this author in PubMed Google Scholar
Clarivate, London, UK.
Breast cancer is the most diagnosed cancer worldwide and accounts for the most cancer-related deaths among women. It is a multifactorial disease that can be categorized into three subtypes according to the hormone receptor (HR) status — oestrogen receptor (ER) and/or progesterone receptor (PR) expression — and human epidermal growth factor receptor 2 (HER2) status. Each subtype determines the prognosis and choice of treatment.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
doi: https://doi.org/10.1038/d41573-024-00133-0
Competing Interests
The authors declare no competing interests
Faculty Position, Center of Excellence in Neuro-Oncology Sciences (CENOS)
Memphis, Tennessee
St. Jude Children's Research Hospital (St. Jude)
Postdoctoral Fellow Position
Birmingham, Alabama (US)
University of Alabama at Birmingham (UAB)
Postdoctoral Scholar - Pharmaceutical Sciences
The University of Tennessee Health Science Center (UTHSC)
Postdoctoral Scholar- Speech Language Pathologist
Postdoctoral fellow phd- cancer biology and metabolism.
Houston, Texas (US)
Baylor College of Medicine (BCM)
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
COMMENTS
Learn about the latest developments in breast cancer screening, treatment, and prevention, funded by the National Cancer Institute. Find out how NCI-supported studies are improving outcomes, reducing disparities, and advancing personalized medicine.
New Strategies and Challenges for Breast Cancer Treatment. 4.1. Emerging Therapies for HR-Positive Breast Cancer. As mentioned in Section 3.1, the major mechanisms of action of current endocrine therapy resistance occur via (1) the mTOR/PI3K/Akt signaling pathway and (2) the actors of the cell cycle progression CDK4/6.
Here, Swain et al. review the current standard of care for HER2+ breast cancer, describe mechanisms of drug resistance and focus on next-generation platforms and therapies for the treatment of ...
Estrogen is already known to fuel breast cancer growth by promoting the proliferation of breast cells. However, the new observations cast this hormone in a different light. They show estrogen is a more central character in cancer genesis because it directly alters how cells repair their DNA. The findings suggest that estrogen-suppressing drugs ...
Breast cancer (BC) is a highly prevalent malignancy worldwide, with complex pathogenesis and treatment challenges. Research reveals that methyltransferase-like 3 (METTL3) is widely involved in the pathogenesis... Dongqiong Xiao, Mingfu Zhang, Yi Qu and Xiaojuan Su. Breast Cancer Research 2024 26 :110.
Breast cancer is the most common cancer in women, but it can also appear in men. Featured AI-assisted detection of lymph node metastases safely reduces costs and time
Read the latest Research articles in Breast cancer from Nature. ... An analysis of 780 breast cancer genomes shows that focal amplifications are frequently preceded by dicentric chromosome ...
Posted: January 20, 2023. Many young women who are diagnosed with early-stage breast cancer want to become pregnant in the future. New research suggests that these women may be able to pause their hormone therapy for up to 2 years as they try to get pregnant without raising the risk of a recurrence in the short term.
An experimental form of immunotherapy that uses an individual's own tumor-fighting immune cells could potentially be used to treat people with metastatic breast cancer, according to results from an ongoing clinical trial led by researchers at the National Cancer Institute's (NCI) Center for Cancer Research, part of the National Institutes of Health.
By Kelley Luckstein. A new multicenter, international study suggests that people who have early-stage triple-negative breast cancer (TNBC) and high levels of immune cells within their tumors may have a lower risk of recurrence and better survival rates even when not treated with chemotherapy. The study was published today in the Journal of American Medical Association (JAMA).
Introduction. Breast cancer is the most commonly diagnosed cancer among female patients and is the leading cause of cancer-related death. 1 There were 300,590 new cases and 43,700 deaths of invasive breast cancer in the United States based on the 2023 prediction, accounting for approximately 30% of female cancers. 1 The treatments of breast cancer include surgery, chemotherapy, radiotherapy ...
Research studies. Current guidance on preventing and treating breast cancer as well as what might cause it (among other things) has come mainly from information discovered from research studies.Research studies can range from studies done in the lab to clinical trials done with hundreds of thousands of people.
Most women who develop breast cancer in a high-income country will survive; the opposite is true for women in most low-income and many middle-income countries . In 2020 breast cancer mortality-to-incidence ratio (MIR) as a representative indicator of 5-year survival rates was 0.30 globally . Taking into consideration the clinical extent of ...
HER2 is a common treatment target for breast cancer. This new drug targets HER3, a biomarker related to HER2, which is associated with poor breast cancer outcomes. About 10% to 20% of newly diagnosed breast cancers are HER2-positive. At the 2023 American Society for Clinical Oncology (ASCO) Annual Meeting, researchers announced positive results ...
Breast Cancer Research is an international, peer-reviewed online journal, publishing original research, reviews, editorials and reports. Open access research articles of exceptional interest are published in all areas of biology and medicine relevant to breast cancer, including normal mammary gland biology, with special emphasis on the genetic, biochemical, and cellular basis of breast cancer.
One patient in the current study, Mary Smrekar, age 55, of Medina, Ohio, said she felt she got a temporary reprieve from certain death. ... Citing rising breast cancer rates in young women, ...
Mortality. The overall breast cancer death rate increased by 0.4% per year from 1975 to 1989, but since has decreased steadily, for a total decline of 43% through 2020. As a result, 460,000 breast cancer deaths were averted in US women from 1989 through 2020.
Risk factors attributable to breast cancer burden. The latest research evidence 1 shown that 44.4% of global cancer deaths and 42.0% of global cancer disability-adjusted life years can be ...
1 INCIDENCE AND EPIDEMIOLOGY OF BREAST CANCER. Female breast cancer has overtaken lung cancer as the most common diagnosed cancer worldwide. The estimated new breast cancer cases reached 2.3 million in 2020, accounting for 11.7% of all new cancers, and 684,996 cases died of it [].In China, breast cancer was the most common malignancy among women, with an estimated number of 306,000 new cases ...
Find out what the latest clinical trial results mean for you. More from this topic. Targeted Therapy 76. Risk Factors 78. Hormonal Therapy 47. Healthcare Disparities 14. Genetic Testing 16. Diagnosis 206. View all 25 topics. 469 Research news. Filter & Sort ... Having a family history of breast cancer and a mutation in any of the five main ...
Sep. 27, 2021 — Researchers suggest that advances in breast cancer prevention research have resulted in new and innovative opportunities to modify breast cancer risk and potentially reduce ...
The ten genes for breast (and ovarian) cancer susceptibility. ATM, BRCA1, BRCA2, CHEK2, PALB2 and TP53 are all established breast cancer susceptibility genes. Over the past 30 years, many other ...
In this paper, we have summarised the predictive factors of radiotherapy sensitivity by reviewing recent research on breast cancer and focused on the following areas: tumor immune microenvironment (TIME), cancer stem cells, noncoding RNAs, signal transduction pathways, genes, etc. This review aims to provide theoretical basis and reference for ...
The 2011 Early Breast Cancer Trialists' Collaborative Group meta-analysis of more than 10 000 women treated with breast-conserving surgery with or without postoperative whole-breast radiotherapy showed that radiotherapy halved the risk of first recurrence (most of which were local) at 10 years, and this translated into a modest reduction in ...
One research letter published in JAMA Oncology last year, for example, suggests GLP-1 drugs might reduce the risk of colon cancer, even among people who are not overweight.
Recent progress in immunobiology has led the way to successful host immunity enhancement against breast cancer. In triple-negative breast cancer, the combination of cancer immunotherapy based on ...
In 2022, his administration set a goal of cutting the death rate from cancer by at least 50 percent by 2047, including by increasing access to early cancer screening and funding research on new ...
In a recent study of over 60,000 adults, those who exercised for two or more hours per week had a 26 percent lower risk of head and neck cancer, a 20 percent lower risk of lung cancer and an 11 ...
Read the latest Research articles in Breast cancer from British Journal of Cancer. ... ER/PR-negative, early or locally advanced breast cancer: A multicenter, randomized, double-blinded, parallel ...
The breast cancer market is forecast to increase 6.7% annually to $50.3 billion in 2032. Fig. 1 | Estimated major-market sales of key therapies for breast cancer, by drug class.