
- Allergy & Immunology
- Anesthesiology
- Critical Care
- Dermatology
- Diabetes & Endocrinology
- Emergency Medicine
- Family Medicine
- Gastroenterology
- General Surgery
- Hematology - Oncology
- Hospital Medicine
- Infectious Diseases
- Internal Medicine
- Multispecialty
- Ob/Gyn & Women's Health
- Ophthalmology
- Orthopedics
- Pathology & Lab Medicine
- Plastic Surgery
- Public Health
- Pulmonary Medicine
- Rheumatology
- Transplantation
- Today on Medscape
- Business of Medicine
- Medical Lifestyle
- Science & Technology
- Medical Students
- Pharmacists

2024 Will See Major Advances in Glaucoma Care
Richard Mark Kirkner
February 08, 2024
Dry eye and glaucoma may be the two most confounding conditions ophthalmologists face. Late last year, the US Food and Drug Administration (FDA) approved three new treatments for dry eye disease (DED) and one new procedure for glaucoma, which means ophthalmologists will soon have the opportunity to incorporate these therapies into their practices. Meanwhile, several investigative treatments for both chronic ailments will continue to move forward.
Undry Those Eyes
Based on a 2022 study in JAMA Ophthalmology , about 27 million Americans have some form of DED or meibomian gland dysfunction. Treatments aim to preserve and enhance tears and tear production to counteract the grittiness and itchiness that accompany DED.

"For decades, we only had one treatment [cyclosporine] for dry eye, then the second one a few years ago, which is lifitegrast, but nothing innovative until very recently," Marjan Farid, MD, director of cornea, cataract and refractive surgery at the Gavin Herbert Eye Institute at the University of California-Irvine, told Medscape Medical News .
"In 2023, I feel that innovation from the pharmaceutical standpoint in this space really exploded, and it's very exciting because dry eye disease is such a multifactorial disease that you can't just go after one angle," said Farid, who is also chair of the American Society of Cataract and Refractive Surgery's cornea clinical committee. "You really need to be able to attack dry eye disease from multiple areas, when the meibomian glands are involved, or whether or not there's blephartitis."
Marjan Farid, MD
You really need to be able to attack dry eye disease from multiple areas, when the meibomian glands are involved, whether there's blephartitis.
The three treatments for DED the FDA approved last year are lotilaner 0.25% ophthalmic solution, which targets the Demodex mites that cause of Demodex blepharitis, a trigger for DED; perfluorohexyloctane ophthalmic solution; and cyclosporine ophthalmic solution 0.1%. The latter two agents coat the ocular surface — perfluorohexyloctane acting as a shield to prevent tear evaporation and cyclosporine 0.1% using perfluorobutylpentane to allow the immunosuppressant cyclosporine to penetrate deeper into the eye.
This year, Farid said, while ophthalmologists will be adopting those treatments, they'll also be watching three emerging treatments poised to report results from clinical trial or take other steps toward FDA approval. They include:
- Selenium sulfide 0.5% ophthalmic ointment will move into phase 3 trials. This ointment is applied directly to the lower eyelid to open the meibomian gland (MGs), secretions from which prevent tear evaporation and tear overflow. Results last year from a phase 2 trial demonstrated improvement in MG secretions in treated patients. "It's a very unique compound because it's the only compound that could potentially open the meibomian gland orifices along lid margin and improve the quality of secretions," Farid said.
- Reproxalap, a reactive aldehyde species (RASP) inhibitor, will be the subject of a new drug application (NDA) resubmission this year. RASPs have been found in elevated levels in ocular and systemic inflammatory disease. The FDA last year notified drug developer Aldeyra Therapeutics that an additional trial was needed to demonstrate efficacy in treating symptoms of DED. Aldeyra said it would resubmit the NDA and report topline trial results in the first half of the year. "That's a really nice anti-inflammatory eye drop that works early in the inflammatory cascade," Farid said. "It acts almost like a steroid does without having the side effects of the steroid."
- AR-15512, a topical transient receptor potential melastatin 8 agonist, may also be the subject of an NDA this year. Topline results from two phase 3 trials last year demonstrated a clinically meaningful increase in tear production.
The Centers for Disease Control and Prevention estimates 3 million Americans have glaucoma. The use of daily eye drops to lower intraocular pressure (IOP) has been a mainstay of glaucoma therapy treatment for decades. However, a 2018 study put the rates of nonadherence as high at 67%.
In part to skirt the adherence issue, several approaches have evolved to lower IOP without relying on drops. They include laser treatments to perforate the eye's trabecular meshwork and improve the outflow of aqueous humor, minimally invasive glaucoma surgery to create a small tunnel or even insert a shunt to enable aqueous outflow, and, more recently, implantable depots that release IOP-lowering drugs within the eye over months.
"Glaucoma is a disease that has a slow onset, so you have to diagnose it as early as possible," Andrew Iwach, MD, a glaucoma specialist in San Francisco and clinical spokesperson for the American Academy of Ophthalmology, told Medscape Medical News . "One challenge with glaucoma is its chronic nature. There are different methods that are being looked at to achieve sustained release of drugs — ways you can implant a little bolus of this medicine," Iwach added.

Glaucoma also requires regular monitoring of changes in IOP, Iwach noted. "During COVID, there was an increased interest in during this remotely,” he said. A remote monitoring platform, Peripherex, was registered last year with the FDA. It consists of a diagnostic online visual field test that can enable patients with glaucoma to provide data on disease changes from home.
A laser platform, the Belkin Eagle Nd:YAG laser for performing selective laser trabeculoplasty (SLT), in December 2023 received FDA clearance. Iwach said this is the first innovation in lasers in 20 years in that it eliminates the need for placing a diagnostic lens on the eye itself to direct the laser pulses, a technique called direct SLT. It uses a computer-driven tacking device.
Looking Ahead
A laser in development is ViaLase, which offers femtosecond laser image-guided high-precision trabeculotomy or FLigHT. The VIA-002 study , which began enrolling patients in September 2023, is comparing ViaLase with SLT to determine reduction in unmedicated IOP at 6 and 12 months. A small feasibility study published last year demonstrated safety of the procedure with an average reduction in IOP of 34.6% at 24 months.
Microshunts inserted into the eye also have been used to reduce IOP. An early stage study is evaluating a new-generation, minimally invasive shunt that, once implanted, allows the ophthalmologist to adjust the level of aqueous outflow in an office-based procedure.
Another December 2023 FDA approval was iDose TR, an implant loaded with the prostaglandin analog travoprost 75 mcg. The implant is scheduled for commercial release in the first quarter of 2024, with a projected wholesale acquisition cost of $13,950 per dose or implant.
Two phase 3 trials compared two iDose TR models with two different travoprost release intervals, defined as the fast- and slow-release iDose TR models, respectively, with topical timolol ophthalmic solution, 0.5% twice a day. The trials demonstrated comparable IOP reduction between all three vehicles. At 12 months, 81% of iDose TR subjects required no IOP-lowering topical medications across both trials.
Also in development is an implant that uses a cilioscleral technique to preserve the anterior chamber of the eye, reducing the risk for complications, such as endothelial cell loss or a filtration bleb, that can occur with other implant procedures. Preliminary results of a 12-month study of 57 patients fitted with a new design with the cilioscleral interpositioning device (CID) showed it lowered IOP an average of 13.9 mmHg vs 15.1 mmHg in earlier studies with the device. In the latest study, more than 85% of patients reported being medication free at 12 months. The CID procedure spares the conjunctiva, requiring only a local incision, according to its developers .
As for topical agents that reduce IOP, cannabinoids may soon find their way into the glaucoma specialist's toolbox. A phase 2 trial evaluating SBI-100 ophthalmic emulsion started enrolling patients late last year. SBI-100 OE is a synthetic prodrug of tetrahydrocannabinol that can bind and activate cannabinoid receptor type 1 in ocular tissues. The trial is scheduled for completion later this year. A phase 1 trial last year demonstrated an average reduction in IOP of 24%.
Another area of focus is on the use of preservatives in topical drops. "One of big issues we're dealing with is preservatives because you're marinating these eyes over years with these drops," Iwach said. Late last year, the first preservative-free form of latanoprost ophthalmic solution 0.005% launched in the United States. Other delivery systems that remove preservatives from topical drops and preservative-free formulations are in the investigative stage, he said.
Farid disclosed financial relationships with Alcon Laboratories, Allergan/AbbVie, Bausch + Lomb, Bio-Tissue, CorneaGen, Harrow, Kala Pharmaceuticals, and Tarsus Pharmaceuticals. Iwach disclosed a previous financial relationship with Belkin Vision as well as relationships with Alcon Laboratories and Innovia.
Richard Mark Kirkner is a medical journalist based in the Philadelphia area.
Send comments and news tips to [email protected] .
TOP PICKS FOR YOU
- Perspective
- Drugs & Diseases
- Global Coverage
- Additional Resources
- What's Medical About Marijuana?
- Ophthalmologists Win Eye Surgery Scope of Practice Battle
- Why Doctors Are Disenchanted With Medicare
- Diseases & Conditions Unilateral Glaucoma
- Diseases & Conditions Neovascular Glaucoma
- Diseases & Conditions Uveitic Glaucoma
- Diseases & Conditions Juvenile Glaucoma
- Hyphema Glaucoma
- Unilateral Glaucoma
- Uveitic Glaucoma
- Phacomorphic Glaucoma
- Malignant Glaucoma
- Juvenile Glaucoma
- Ocular Trauma: 8 Potentially Devastating Eye Injuries
- FDA Approves Implant for Glaucoma
- A Better Way to Assess Glaucoma?
- Stiff Arteries Can Predict Risk for Glaucoma
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

Alzheimer’s drug may save lives through ‘suspended animation’

Implantable device responds to opioid overdose
Study detects ‘hidden consciousness’ in brain injury patients

Researchers at HMS have successfully restored vision loss and reversed glaucoma-induced damage in mice.
Credit: Sinclair Lab/Harvard Medical School
Seeing clearly again
Ryan Jaslow
MEEI Communications
Harvard Medical School scientists reverse age-related vision loss, eye damage from glaucoma in mice
Harvard Medical School scientists report they have successfully restored vision in mice by turning back the clock on aged eye cells in the retina to recapture youthful gene function.
The team’s work, described Dec. 2 in the publication Nature, represents the first demonstration that it may be possible to safely reprogram complex tissues, such as the nerve cells of the eye, to an earlier age.
In addition to resetting the cells’ aging clock, the researchers successfully reversed vision loss in animals with a condition mimicking human glaucoma, a leading cause of blindness around the world.
The achievement represents the first successful attempt to reverse glaucoma-induced vision loss, rather than merely stem its progression, the team said.
If replicated through further studies, the approach could pave the way for therapies to promote tissue repair across various organs and reverse aging and age-related diseases in humans.
“Our study demonstrates that it’s possible to safely reverse the age of complex tissues such as the retina and restore its youthful biological function,” said senior author David Sinclair, professor of genetics in the Blavatnik Institute at Harvard Medical School, co-director of the Paul F. Glenn Center for Biology of Aging Research at HMS and an expert on aging.
Sinclair and colleagues caution that the findings remain to be replicated in further studies, including in different animal models, before any human experiments. Nonetheless, they add, the results offer a proof of concept and a pathway to designing treatments for a range of age-related human diseases.
“If affirmed through further studies, these findings could be transformative for the care of age-related vision diseases like glaucoma and to the fields of biology and medical therapeutics for disease at large,” Sinclair said.
“At the beginning of this project, many of our colleagues said our approach would fail or would be too dangerous to ever be used. Our results suggest this method is safe and could potentially revolutionize the treatment of the eye and many other organs affected by aging.” Yuancheng Lu, lead study author
For their work, the team used an adeno-associated virus (AAV) as a vehicle to deliver into the retinas of mice three youth-restoring genes — Oct4, Sox2, and Klf4 — that are normally switched on during embryonic development. The three genes, together with a fourth one, which was not used in this work, are collectively known as Yamanaka factors.
The treatment had multiple beneficial effects on the eye. First, it promoted nerve regeneration following optic-nerve injury in mice with damaged optic nerves. Second, it reversed vision loss in animals with a condition mimicking human glaucoma. And third, it reversed vision loss in aging animals without glaucoma.
The team’s approach is based on a new theory about why we age. Most cells in the body contain the same DNA molecules but have widely diverse functions. To achieve this degree of specialization, these cells must read only genes specific to their type. This regulatory function is the purview of the epigenome, a system of turning genes on and off in specific patterns without altering the basic underlying DNA sequence of the gene.
This theory postulates that changes to the epigenome over time cause cells to read the wrong genes and malfunction — giving rise to diseases of aging. One of the most important changes to the epigenome is DNA methylation, a process by which methyl groups are tacked onto DNA. Patterns of DNA methylation are laid down during embryonic development to produce the various cell types. Over time, youthful patterns of DNA methylation are lost, and genes inside cells that should be switched on get turned off and vice versa, resulting in impaired cellular function. Some of these DNA methylation changes are predictable and have been used to determine the biologic age of a cell or tissue.
Yet, whether DNA methylation drives age-related changes inside cells has remained unclear. In the current study, the researchers hypothesized that if DNA methylation does, indeed, control aging, then erasing some of its footprints might reverse the age of cells inside living organisms and restore them to their earlier, more youthful state.
Past work had achieved this feat in cells grown in laboratory dishes but fell short of demonstrating the effect in living organisms.
The new findings demonstrate that the approach could be used in animals as well.
Overcoming an important hurdle
Lead study author, Yuancheng Lu , research fellow in genetics at HMS and a former doctoral student in Sinclair’s lab, developed a gene therapy that could safely reverse the age of cells in a living animal.
Lu’s work builds on the Nobel Prize winning discovery of Shinya Yamanaka, who identified the four transcription factors, Oct4, Sox2, Klf4, c-Myc, that could erase epigenetics markers on cells and return these cells to their primitive embryonic state from which they can develop into any other type of cell.
Subsequent studies, however, showed two important setbacks. First, when used in adult mice, the four Yamanaka factors could also induce tumor growth, rendering the approach unsafe. Second, the factors could reset the cellular state to the most primitive cell state, thus completely erasing a cell’s identity.
Lu and colleagues circumvented these hurdles by slightly modifying the approach. They dropped the gene c-Myc and delivered only the remaining three Yamanaka genes, Oct4, Sox2, and Klf4. The modified approach successfully reversed cellular aging without fueling tumor growth or losing their identity.
Gene therapy applied to optic nerve regeneration
In the current study, the researchers targeted cells in the central nervous system because it is the first part of the body affected by aging. After birth, the ability of the central nervous system to regenerate declines rapidly.
To test whether the regenerative capacity of young animals could be imparted to adult mice, the researchers delivered the modified three-gene combination via an AAV into retinal ganglion cells of adult mice with optic nerve injury.
For the work, Lu and Sinclair partnered with Zhigang He , HMS professor of neurology and of ophthalmology at Boston Children’s Hospital, who studies optic nerve and spinal cord neuro-regeneration.
The treatment resulted in a two-fold increase in the number of surviving retinal ganglion cells after the injury and a five-fold increase in nerve regrowth.
“At the beginning of this project, many of our colleagues said our approach would fail or would be too dangerous to ever be used,” said Lu. “Our results suggest this method is safe and could potentially revolutionize the treatment of the eye and many other organs affected by aging.”
Reversal of glaucoma and age-related vision loss
More like this.

Focusing on the fovea

Linking sight and movement
Making sense of how the blind ‘see’ color
Following the encouraging findings in mice with optic nerve injuries, the team partnered with colleagues at Schepens Eye Research Institute of Massachusetts Eye and Ear Bruce Ksander , HMS associate professor of ophthalmology, and Meredith Gregory-Ksander , HMS assistant professor of ophthalmology. They planned two sets of experiments: one to test whether the three-gene cocktail could restore vision loss due to glaucoma and another to see whether the approach could reverse vision loss stemming from normal aging.
In a mouse model of glaucoma, the treatment led to increased nerve cell electrical activity and a notable increase in visual acuity, as measured by the animals’ ability to see moving vertical lines on a screen. Remarkably, it did so after the glaucoma-induced vision loss had already occurred.
“Regaining visual function after the injury occurred has rarely been demonstrated by scientists,” Ksander said. “This new approach, which successfully reverses multiple causes of vision loss in mice without the need for a retinal transplant, represents a new treatment modality in regenerative medicine.”
The treatment worked similarly well in elderly, 12-month-old mice with diminishing vision due to normal aging. Following treatment of the elderly mice, the gene expression patterns and electrical signals of the optic nerve cells were similar to young mice, and vision was restored. When the researchers analyzed molecular changes in treated cells, they found reversed patterns of DNA methylation — an observation suggesting that DNA methylation is not a mere marker or a bystander in the aging process, but rather an active agent driving it.
“What this tells us is the clock doesn’t just represent time — it is time,” said Sinclair. “If you wind the hands of the clock back, time also goes backward.”
The researchers said that if their findings are confirmed in further animal work, they could initiate clinical trials within two years to test the efficacy of the approach in people with glaucoma. Thus far, the findings are encouraging, researchers said. In the current study, a one-year, whole-body treatment of mice with the three-gene approach showed no negative side effects.
Other authors on the paper include Benedikt Brommer, Xiao Tian, Anitha Krishnan, Margarita Meer, Chen Wang, Daniel Vera, Qiurui Zeng, Doudou Yu, Michael Bonkowski, Jae-Hyun Yang, Songlin Zhou, Emma Hoffmann, Margarete Karg, Michael Schultz, Alice Kane, Noah Davidsohn, Ekaterina Korobkina, Karolina Chwalek, Luis Rajman, George Church, Konrad Hochedlinger, Vadim Gladyshev, Steve Horvath, and Morgan Levine.
This work was supported in part by a Harvard Medical School Epigenetics Seed Grant and Development Grant, The Glenn Foundation for Medical Research, Edward Schulak, the National Institutes of Health (grants R01AG019719,R37AG028730, R01EY026939, R01EY021526, R01AG067782, R01GM065204, R01AG065403, R01EY025794, R24EY028767 and R21EY030276), and the St. Vincent de Paul Foundation.
Share this article
You might like.
Could buy patients more time to survive critical injuries and diseases, even when disaster strikes far from a hospital
Without assistance, it allows for precise administration of naloxone at the moment it is needed
25% of participants with severe brain injury followed instructions covertly
Good genes are nice, but joy is better
Harvard study, almost 80 years old, has proved that embracing community helps us live longer, and be happier
Examining new weight-loss drugs, pediatric bariatric patients
Researcher says study found variation in practices, discusses safety concerns overall for younger users
Shingles may increase risk of cognitive decline
Availability of vaccine offers opportunity to reduce burden of shingles and possible dementia
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
News releases.
News Release
Thursday, July 22, 2021
Scientists discover gene therapy provides neuroprotection to prevent glaucoma vision loss
An NIH-funded research project found that calcium modulator CaMKII protects the optic nerve in mice, opening the door to new sight-saving therapy.

A form of gene therapy protects optic nerve cells and preserves vision in mouse models of glaucoma, according to research supported by NIH’s National Eye Institute. The findings suggest a way forward for developing neuroprotective therapies for glaucoma, a leading cause of visual impairment and blindness. The report was published in Cell.
Glaucoma results from irreversible neurodegeneration of the optic nerve, the bundle of axons from retinal ganglion cells that transmits signals from the eye to the brain to produce vision. Available therapies slow vision loss by lowering elevated eye pressure, however some glaucoma progresses to blindness despite normal eye pressure. Neuroprotective therapies would be a leap forward, meeting the needs of patients who lack treatment options.
“Our study is the first to show that activating the CaMKII pathway helps protect retinal ganglion cells from a variety of injuries and in multiple glaucoma models,” said the study’s lead investigator, Bo Chen, Ph.D., associate professor of ophthalmology and neuroscience at the Icahn School of Medicine at Mount Sinai in New York City.
The CaMKII (calcium/calmodulin-dependent protein kinase II) pathway regulates key cellular processes and functions throughout the body, including retinal ganglion cells in the eye. Yet the precise role of CaMKII in retinal ganglion cell health is not well understood. Inhibition of CaMKII activity, for example, has been shown to be either protective or detrimental to retinal ganglion cells, depending on the conditions.
Using an antibody marker of CaMKII activity, Chen’s team discovered that CaMKII pathway signaling was compromised whenever retinal ganglion cells were exposed to toxins or trauma from a crush injury to the optic nerve, suggesting a correlation between CaMKII activity and retinal ganglion cell survival.
Searching for ways to intervene, they found that activating the CaMKII pathway with gene therapy proved protective to the retinal ganglion cells. Administering the gene therapy to mice just prior to the toxic insult (which initiates rapid damage to the cells), and just after optic nerve crush (which causes slower damage), increased CaMKII activity and robustly protected retinal ganglion cells.
Among gene therapy-treated mice, 77% of retinal ganglion cells survived 12 months after the toxic insult compared with 8% in control mice. Six months following optic nerve crush, 77% of retinal ganglion cells had survived versus 7% in controls.
Similarly, boosting CaMKII activity via gene therapy proved protective of retinal ganglion cells in glaucoma models based on elevated eye pressure or genetic deficiencies.
Increasing retinal ganglion cell survival rates translated into greater likelihood of preserved visual function, according to cell activity measured by electroretinogram and patterns of activity in the visual cortex.
Three vision-based behavioral tests also confirmed sustained visual function among the treated mice. In a visual water task, the mice were trained to swim toward a submerged platform on the basis of visual stimuli on a computer monitor. Depth perception was confirmed by a visual cliff test based on the mouse’s innate tendency to step to the shallow side of a cliff. Lastly, a looming test determined that treated mice were more apt to respond defensively (by hiding, freezing or tail rattling) when shown an overhead stimulus designed to simulate a threat, compared with untreated mice.
“If we make retinal ganglion cells more resistant and tolerant to the insults that cause cell death in glaucoma, they might be able to survive longer and maintain their function,” Chen concluded.
This study was supported by NEI grants R01EY028921, R01 EY024986. NEI is part of the National Institutes of Health.
For more information about glaucoma, visit https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/glaucoma
This press release describes a basic research finding. Basic research increases our understanding of human behavior and biology, which is foundational to advancing new and better ways to prevent, diagnose, and treat disease. Science is an unpredictable and incremental process— each research advance builds on past discoveries, often in unexpected ways. Most clinical advances would not be possible without the knowledge of fundamental basic research.
NEI leads the federal government’s research on the visual system and eye diseases. NEI supports basic and clinical science programs to develop sight-saving treatments and address special needs of people with vision loss. For more information, visit https://www.nei.nih.gov .
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
NIH…Turning Discovery Into Health ®
Guo X, Zhou J, Starr C, Mohns EJ, Li Y, Chen E, Yoon Y, Kellner CP, Tanaka K, Wang H, Liu W, LR, Demb JB, Crair MC, and Chen B. “Preservation of vision after CaMKII-mediated protection of retinal ganglion cells.” Published online July 22, 2021 in Cell. DOI:10.1016/j.cell.2021.06.031
Connect with Us
- More Social Media from NIH

Enter a Search Term
Beyond eye pressure: a potential new path for treating glaucoma.
- Research News
Scientifically reviewed by: Preeti Subramanian, PhD
A team of National Glaucoma Research (NGR) scientists has linked a genetic mutation with vision damage in glaucoma that points to the possibility of an entirely new treatment method targeting specialized glial cells. The discovery, published in Stem Cell Reports , could work in addition to the standard glaucoma treatment approach of using eye drops to control the rise in fluid pressure that might otherwise damage the optic nerve. In experiments, Jason Meyer, PhD , and Cátia Gomes, PhD , both at Indiana University School of Medicine—discovered that astrocytes with a mutation associated with glaucoma grew dysfunctional in ways that damaged axons and contributed to neurodegeneration. Conversely, introducing healthy astrocytes via adult stem cell techniques rescued some of these neurodegenerative features in cells.
Astrocytes are a type of glial cells—the most prevalent cell type in the central nervous system—and play a supporting role to other tissue. In the eye, they help to nourish and maintain the health of neurons, including retinal ganglion cells (RGCs) that are damaged or destroyed in glaucoma. These cells have the important role of carrying light signals from the eye to the brain; these signals are carried over long “tails” that extend all the way from RGC bodies to the brain. Axons leave the eye in a bundle that’s collectively referred to as the optic nerve.
The chief explanation for glaucoma is a chronic pressure build-up in fluids of the eye that over time damages cells and the eye’s drainage pathways. A remaining riddle, however, is why glaucoma can worsen, even when pressure is well controlled, and why glaucoma is sometimes diagnosed in eyes with normal pressure.
The Meyer lab has long focused on using adult stem cell techniques to replicate eye tissue in the lab. In the short term, they’re creating realistic new models to study glaucoma, and that’s what led to this recent discovery.
“When we turn these donated cells into stem cells, they become a very powerful model for us to study the disease in a dish. We look at the cells in close detail—long before a patient would develop symptoms— and ask, “What's leading to those early changes?” Dr. Meyer said.
Someday, his team hopes to use cells and surrounding tissues generated this way to replace vision loss from glaucoma. As part of that larger effort, this recent work is the first to use adult stem cells derived from glaucoma patients to examine the specific ways that astrocytes contribute to glaucoma. The results chart new ground, showing that astrocytes may offer a promising target for therapeutic intervention.
As a result, “we're hopefully getting toward a more holistic approach [to glaucoma],” Dr. Meyer said. As such, the field is moving beyond studies of eye pressure and other factors directly related to RGC death to explore how surrounding cells can undergo changes to make a condition worse.
“We can now start addressing some of these problems, not just by one approach, but by multiple approaches, and hopefully get to therapeutics or cures a lot faster,” he said.
Can Stem Cell Treatments Cure My Glaucoma? (Expert Article)
Lifestyle Changes for Glaucoma Patients (Expert Article)
About BrightFocus Foundation
BrightFocus Foundation is a premier nonprofit funder of research to defeat Alzheimer’s, macular degeneration, and glaucoma. Through its flagship research programs—Alzheimer’s Disease Research, National Glaucoma Research, and Macular Degeneration Research—the Foundation is currently supporting a $75 million portfolio of 287 scientific projects. BrightFocus has awarded nearly $275 million in groundbreaking medical research funding since inception and shares the latest research findings, expert information, and English/Spanish disease resources to empower the millions impacted by these devastating diseases. Join our community at brightfocus.org .
The information provided in this section is a public service of BrightFocus Foundation, should not in any way substitute for the advice of a qualified healthcare professional, and is not intended to constitute medical advice. Although we make efforts to keep the medical information on our website updated, we cannot guarantee that the information on our website reflects the most up-to-date research. Please consult your physician for personalized medical advice; all medications and supplements should only be taken under medical supervision. BrightFocus Foundation does not endorse any medical product or therapy.
Some of the content in this section is adapted from other sources, which are clearly identified within each individual item of information.
Don't miss out.
Receive glaucoma breakthrough news, research updates, and inspiring stories.
More From Forbes
A promising new approach for glaucoma.
- Share to Facebook
- Share to Twitter
- Share to Linkedin
Eye looking at camera
This story is part of a series on the current progression in Regenerative Medicine. This piece is part of a series dedicated to the eye and improvements in restoring vision.
In 1999, I defined regenerative medicine as the collection of interventions that restore tissues and organs damaged by disease, injured by trauma, or worn by time to normal function. I include a full spectrum of chemical, gene, and protein-based medicines, cell-based therapies, and biomechanical interventions that achieve that goal.
Glaucoma is a debilitating eye ailment that can cause irreversible harm to the optic nerve and eventually result in loss of vision. The condition is often asymptomatic in its early stages, making it challenging to diagnose and treat . It occurs when the fluid pressure inside the eye increases, damaging the optic nerve and causing vision loss. Fortunately, recent medical breakthroughs in RGC (retinal ganglion cell) replacement therapy offer hope for those suffering from this condition. This revolutionary treatment involves transplanting healthy RGCs to replace the damaged ones and restore visual function. Doing so aims to prevent further damage to the optic nerve and potentially reverse the damage already done. With this new treatment, there is hope for individuals with glaucoma.
What are Retinal Ganglion Cells?
Retinal Ganglion Cells (RGCs) are specialized neurons that play a critical role in the visual system by transmitting information from the retina to the brain via the optic nerve. These highly complex cells comprise more than a dozen molecularly, functionally, and topographically unique subtypes. Each subtype has distinct morphology, connectivity, and response properties, allowing it to perform different visual system functions.
RGCs are the final output neurons of the retina. They are responsible for encoding visual information into electrical signals sent to the brain. These signals are then processed by higher visual centers in the brain, which enables us to see and perceive the world around us.
Despite their complexity, RGCs have been a promising target for therapeutic intervention as they do not regenerate naturally once they deteriorate. Any damage to these cells can result in permanent vision loss. Scientists are actively working to develop new therapies that can help protect and restore RGCs in individuals with retinal degenerative diseases such as glaucoma and age-related macular degeneration.
Best High-Yield Savings Accounts Of 2024
Best 5% interest savings accounts of 2024, stem cells & organoids for glaucoma.
Stem cell-based transplantation has emerged as a viable option for replacing lost or damaged retinal ganglion cells (RGCs). As stem cells can differentiate into various cell types, including RGCs, they present a potential source for RGC replacement therapy. However, the limited availability of RGCs in stem cell-derived cultures makes the integration of RGCs into the host retina a complex task. Organoids offer a promising alternative to traditional branch cell-based transplantation methods.
Organoids are self-organizing three-dimensional structures that closely resemble the complexity and organization of the retina. They are generated by culturing stem cells in a controlled environment that mimics the developmental process of the retina. Recent advances in organoid protocols have made it possible to create RGCs from both human and mouse stem cells, overcoming the limitations of traditional stem cell-based transplantation methods.
Methods of Retinal Cell Replacement
Cell delivery can be either suprachoroidal, intravitreal or subretinal.
Subretinal space and vitreous cavity transplantation are two potential methods for RGC replacement. Subretinal space transplantation involves transplanting cells into the subretinal space, which is located between the retinal pigment epithelium and the photoreceptor layer. The transplanted cells can integrate into the host retina and potentially restore vision.
Vitreous cavity transplantation, on the other hand, involves transplanting cells into the vitreous cavity of the eye, which is the gelatinous substance located behind the lens. The transplanted cells can secrete neurotrophic factors that promote the survival of the remaining RGCs and stimulate the regeneration of damaged ones.
Clinical Trials Exploring Cell Therapies
Numerous studies are currently investigating the possibility of using RGC replacement therapy as a treatment for glaucoma. One such study was conducted on rats with glaucoma, and it showed significant improvement in visual function and RGC survival when RGC-like cells were transplanted into them. These RGC-like cells were differentiated from human-induced pluripotent stem cells, which can develop into any cell in the human body.
The study demonstrated that human-induced pluripotent stem cells could be a potential source for RGC transplantation in glaucoma patients. However, further research is necessary to determine the safety and effectiveness of this therapy before it can be implemented in clinical practice.
The Challenges of Making Retinal Ganglion Cells a Treatment
Still, despite the promise of studies, there are some challenges. A review done by a team in Spain assessed these challenges and more. One of the significant challenges is understanding the origin of RGCs and how to replicate their natural development in laboratory settings.
Another area for improvement is the difficulty in scaling up the production of RGCs and ensuring they can be produced in large quantities. Additionally, once produced, there are challenges in integrating and ensuring the survival of transplanted RGCs in the host tissue.
Regrowing RGC axons is also a significant challenge, as it requires identifying the factors that promote axon growth and determining how to apply them effectively. Finally, achieving functional RGC replacement is a significant hurdle, as it involves creating RGCs that can function as well as, or better than, the RGCs they are replacing.
This illustrative graph displays three commonly used types/formats of donor cells in retinal ... [+] ganglion cell (RGC) replacement studies.
However, recent advances in stem cell technologies and organoid protocols discussed before offer new solutions to these limitations. For example, organoid protocols enable the generation of RGCs that more closely resemble those found in the human retina, thus improving the accuracy and efficacy of transplantation. Additionally, stem cell technologies have been used to create RGCs capable of integrating into the host tissue and regenerating axons.
RGC replacement therapy shows potential as a new therapeutic approach for treating glaucoma. Although there are challenges to overcome regarding scaling up RGC production and achieving reliable and functional integration, recent advancements in stem cell technologies and organoid protocols offer promising solutions. Clinical trials investigating the potential of RGC replacement therapy have reported positive outcomes, providing hope for restoring vision in glaucoma patients.
To learn more about the eye, read more stories at www.williamhaseltine.com
- Editorial Standards
- Reprints & Permissions
New research aims to develop novel therapeutic for glaucoma
Researchers at Indiana University School of Medicine are using a novel approach to hopefully develop a new therapy for glaucoma, a complex disease that eventually leads to blindness, thanks to a new five-year, $2 million R01 grant from the National Eye Institute.
The project, led by Tasneem Sharma, Ph.D. is called “Therapeutic Intervention to Target Human Glaucoma Pathogenesis.” It focuses on providing a foundation for developing a new glaucoma therapeutic by testing human neurons and a regenerative therapy to rescue visual neurons from dying preclinically in human eyes under glaucoma conditions. This combination has never been used before.
Sharma hopes the results of this research project will lead to new clinical trials for glaucoma patients to study the effectiveness of potential new treatments.
A new treatment for glaucoma?
- Feinberg School of Medicine
- Global Health
A Northwestern Medicine study in mice has identified new treatment targets for glaucoma, including preventing a severe pediatric form of glaucoma, as well as uncovering a possible new class of therapy for the most common form of glaucoma in adults.
In people with high pressure glaucoma, fluid in the eye doesn’t properly drain and builds up pressure on the optic nerve, leading to vision loss. It affects 60 million people worldwide and is the most common cause of blindness in people over 60 years old.
While there are a few treatments available for open angle glaucoma, the most common form of glaucoma in adults (eye drops, oral medication, laser treatments), there are no cures, and a severe form of glaucoma in children between birth and three years old known as primary congenital glaucoma can only be treated with surgery.
“Although primary congenital glaucoma is much rarer than open angle glaucoma , it is devastating for children,” said corresponding author Dr. Susan Quaggin , chief of nephrology and hypertension in the Department of Medicine at Northwestern University Feinberg School of Medicine. “New treatments and new classes of treatments are urgently needed to slow vision loss in both forms.
Using gene editing, the scientists in the study developed new models of glaucoma in mice that resembled primary congenital glaucoma. By injecting a new, long-lasting and non-toxic protein treatment (Hepta-ANGPT1) into mice, the scientists were able to replace the function of genes that, when mutated, cause glaucoma. With this injectable treatment, the scientists also successfully prevented glaucoma from ever forming in one model. This same therapy, when injected into the eyes of healthy adult mice, reduced pressure in the eyes, supporting it as a possible new class of therapy for the most common cause of glaucoma in adults (high intraocular pressure open angle glaucoma).
The study, “Cellular crosstalk regulates the aqueous humor outflow pathway and provides new targets for glaucoma therapies,” was published Oct. 18 in the journal Nature Communications.
60 million people worldwide are affected by glaucoma
The next step is to develop the appropriate delivery system for the successful new protein treatment in patients and bring it to production, Quaggin said.
Additionally, the scientists used bioinformatics and single cell RNA sequence data to understand and identify glaucoma pathways that can be explored in the future for additional therapeutic targets for the disease, such as ones that regulate communication with a specialized blood vessel in the eye (Schlemm’s canal) that is important for draining fluid and maintaining normal eye pressure.
“Having a treatment that can promote remodeling and/or growth of a defective Schlemm’s canal to treat glaucoma would be fantastic,” Quaggin said. “These studies are the first step to that goal.
“Our hope is that this study leads to the first targeted therapy that effectively promotes (aqueous humor) fluid outflow from the front of an eye, reversing the underlying biologic defect in patients with glaucoma.”
Other Northwestern co-authors are Ben Thompson (first), Dr. Jing Jin , Pan Liu and medical student Raj Purohit. This study builds on major teamwork and an ongoing collaboration with University of Madison-Wisconsin co-authors Terri Young and Stuart Thomson.
Funding for the study was provided by the National Eye Institute (grant numbers R01 EY025799, P30 EY016665 and R01 EY014685), the National Heart, Lung and Blood Institute (grant number R01 HL124120), the National Institutes of Health Office of the Director (grant number 1S10OD025120), the National Institute of Diabetes and Digestive and Kidney Diseases (grant number P30 DK114857), the National Cancer Institute (grant number CCSG P30 CA060553), and Research to Prevent Blindness.
Editor’s Picks

Construction crews work to protect Northwestern’s South Campus shoreline
New biomaterial regrows damaged cartilage in joints, chronicling chicago, one column at a time, related stories.

HIV prevention medication users fear being stigmatized as ‘promiscuous’
Neurological symptoms in long covid patients persist up to three years, cancerous uterine tumors more aggressive in black patients than white patients.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Mayo Clin Proc Innov Qual Outcomes
- v.6(6); 2022 Dec
Updates on the Diagnosis and Management of Glaucoma
Glaucoma is the leading cause of blindness throughout the world (after cataracts); therefore, general physicians should be familiar with the diagnosis and management of affected patients. Glaucomas are usually categorized by the anatomy of the anterior chamber angle (open vs narrow/closed), rapidity of onset (acute vs chronic), and major etiology (primary vs secondary). Most glaucomas are primary (ie, without a contributing comorbidity); however, several coexisting ophthalmic conditions may serve as the underlying etiologies of secondary glaucomas. Chronic glaucoma occurs most commonly; thus, regular eye examinations should be performed in at-risk patients to prevent the insidious loss of vision that can develop before diagnosis. Glaucoma damages the optic nerve and retinal nerve fiber layer, leading to peripheral and central visual field defects. Elevated intraocular pressure (IOP), a crucial determinant of disease progression, remains the only modifiable risk factor; thus, all current treatments (medications, lasers, and operations) aim to reduce the IOP. Pharmacotherapy is the usual first-line therapy, but noncompliance, undesirable adverse effects, and cost limit effectiveness. Laser and surgical treatments may lower IOP significantly over long periods and may be more cost effective than pharmacotherapy, but they are plagued by greater procedural risks and frequent treatment failures. Traditional incisional procedures have recently been replaced by several novel, minimally invasive glaucoma surgeries with improved safety profiles and only minimal decreases in efficacy. Minimally invasive glaucoma surgeries have dramatically transformed the surgical management of glaucoma; nevertheless, large, randomized trials are required to assess their long-term efficacy.
Article Highlights
- • Glaucoma, a leading cause of blindness throughout the world, presents with an open or closed anterior chamber angle, structural damage to the optic nerve (seen in all stages), and visual field defects (seen in later stages). Glaucoma may be asymptomatic until the late stages, thereby emphasizing the need for general physicians to understand important diagnostic criteria and management options.
- • The progression of glaucoma is mitigated by lowering the intraocular pressure, which is done with topical medications, laser procedures, or incisional operations.
- • Minimally invasive glaucoma surgery, with a favorable safety profile and efficacy that rivals traditional incisional procedures, has transformed glaucoma care.
Glaucoma can be defined as a progressive optic neuropathy that induces optic disc cupping and retinal ganglion cell apoptosis. 1 As the world’s leading cause of irreversible blindness, the disease currently affects 3.5% of individuals aged between 40 and 80 years. The incidence of glaucoma is increasing, together with life expectancies, in resource-limited countries, and nearly 112 million people are expected to be affected by 2040. 1 , 2 Early detection can slow disease progression, but because visual field loss may be asymptomatic until the late stages, a timely diagnosis is frequently delayed. 3 Common risk factors for glaucoma include advancing age, a positive family history, race (non-Caucasian), and elevated intraocular pressure (IOP). 4 , 5 Once diagnosed with glaucoma, most patients require lifelong care.
Aqueous humor is produced by the ciliary body, and after percolating through the posterior chamber, around the lens, and through the pupil, it exits the eye through the semiporous trabecular meshwork (TM) in the iridocorneal angle of the anterior chamber. Aqueous humor then flows into the circumferential vascular collection duct (Schlemm canal) and leaves the eye through the distal collector channels that drain into the episcleral venous system. 6 , 7 , 8 A detailed anatomical view of the anterior eye segment and the aqueous outflow pathway is displayed in Figure 1 . The pathogenesis of glaucoma includes inadequate drainage of aqueous humor because of increased resistance through the meshwork 7 or occlusion of the angle, 9 both of which elevate the IOP. Elevated IOP contributes to an irreversible, progressive ocular neuropathy characterized by retinal ganglion cell apoptosis. 1 Patients with elevated IOP without other signs of glaucoma are said to have ocular hypertension, and those with optic disc enlargement but normal IOP and no other signs of glaucoma are classified as glaucoma suspects.

Ultrasound biomicroscopy (UBM) of the anterior eye segment. A, UBM shows the ciliary zonules (CZ), ciliary body (CB), sclera (S), cornea (C), anterior chamber (AC), posterior chamber (PC), and lens (L). The anterior chamber angle (ACA) is indicated by the arrow. B, Magnified UBM of the ACA shows the trabecular meshwork (TM), Schlemm canal (SC), and collector channels (CCs).
The risk factors and pathogenesis that underly glaucoma have been well described in the literature; however, the biological basis of the disease remains incompletely understood. The biomechanical and vascular theories of glaucoma propose that elevated IOP compromises axonal integrity at the optic nerve head (ONH), which leads to ganglion cell apoptosis. 5 The biomechanical theory posits that abnormally narrow scleral fenestrations at the ONH limit axoplasmic flow, 5 , 7 , 10 whereas the vascular theory states that decreased perfusion pressure leads to hypoxia and ischemic damage of the ONH. 5 , 7 , 11 Both theories include IOP as a risk factor; however, one-third of patients with glaucoma have normal IOPs (normal tension glaucoma). 5 Glaucoma has been associated with Alzheimer disease 12 and a loss of cognitive function, 13 which suggests that neurodegeneration may contribute to the pathogenesis. 5 However, despite the different pathogenetic theories, elevated IOP consistently contributes to disease progression and remains the only treatable risk factor. 5 , 7
The goal of glaucoma treatment is to lower IOP with medications, laser procedures, and/or operation. First-line therapy is usually pharmacotherapy, with laser and surgical procedures added for further IOP reduction in eyes with inadequate initial responses. Incisional operations consist of filtration procedures (eg, trabeculectomy) or tube shunt implantation, both of which reroute aqueous humor flow past the damaged angle into the subconjunctival space forming a filtration bleb. 14
Traditional incisional operations lower the IOP effectively; however, complication rates, including scar tissue proliferation, endophthalmitis, and conjunctival hemorrhage, are high. The IOP-lowering effect often decreases over time, which results in high 5-year reoperation rates (trabeculectomy, 15.1%; tube shunt implantation, 14.0%; EX-PRESS shunt, 18.3%). 15 , 16 , 17 These high reoperation rates speak to the need for procedures that increase conventional aqueous outflow while protecting the conjunctiva from surgical manipulation. This has led to the development of several conjunctival sparing, minimally invasive glaucoma surgeries (MIGSs) for the treatment of primary open-angle glaucoma (POAG). Minimally invasive glaucoma surgeries do not reduce IOP as well as traditional filtering procedures, but they have excellent safety profiles. 18
We believe that because of the expanding treatment options and increasing worldwide prevalence of glaucoma, an updated commentary on glaucoma and its treatment options is important for medical physicians. In this article, we provide a comprehensive updated review of the diagnosis and management of adult glaucoma through 2022.
A broad literature search with no time frame was carried out in PubMed with the following key words: “glaucoma prevalence,” “glaucoma risk factors,” “glaucoma diagnosis,” “glaucoma management,” “open-angle glaucoma,” angle-closure glaucoma,” “secondary glaucoma,” “tonometry,” “glaucoma medication,” “conventional aqueous outflow,” “unconventional aqueous outflow,” “glaucoma laser procedures,” “trabeculectomy,” “glaucoma tube shunt surgery,” and “minimally invasive glaucoma surgery.” Identified articles and their references were scrutinized, and those relevant to the subject matter were selected.
Diagnosis of Glaucoma
Types of glaucoma.
Glaucoma may be broadly categorized as open-angle glaucoma (OAG) and angle-closure glaucoma (ACG). Primary OAG and primary ACG are seen most frequently; however, several ocular conditions cause secondary glaucomas ( Table 1 ).
Table 1
Common Glaucoma Types are Listed According to Whether the Anterior Chamber Angle is Open or Closed a
| Glaucoma type | Clinical features |
|---|---|
| Open-angle glaucoma | Normal iridocorneal angle; no iris occlusion |
| Primary open angle (includes normal tension glaucoma) | |
| Pigmentary | |
| Exfoliative | |
| Uveitic | |
| Traumatic | |
| Induced by steroids | |
| Induced by antineoplastic drugs | |
| Induced by increased episcleral venous pressure | |
| Angle-closure glaucoma | Closed iridocorneal angle; iris occlusion |
| Primary angle closure | |
| Neovascular | |
| Phacomorphic | |
| Induced by iridocorneal endothelial syndrome | |
| Induced by iris tumor/ciliary body tumor/Soemmering ring | |
| Induced by medications |
Most eyes with glaucoma have diminished conventional aqueous outflow despite a normal gonioscopic appearance of the iridocorneal angle. These OAGs are slowly progressive optic neuropathies in which ONH cupping gradual increases and peripheral visual field loss develops. 15 , 19 The most common type of glaucoma—the POAG—affects 74% of patients with glaucoma. 20 Outflow resistance may be modulated by hydrodynamic pore-substrate interactions within the inner wall of the Schlemm canal, and patients with POAG have been found to have reduced pore density. 21
Several types of secondary OAG occur much less frequently than POAG. Pigmentary glaucoma occurs when friction between the lens zonules and iris pigment epithelium releases pigment granules that lodge in the TM and increase outflow resistance. 22 , 23 Exfoliative glaucoma, the most common form of secondary OAG, occurs when microscopic clumps of protein fibers are synthesized within the eye and clog the TM. 24 Exfoliation material has also been found in the heart, kidney, liver, and lungs. 24 , 25 Other forms of secondary OAG include uveitic and traumatic glaucomas, 26 , 27 , 28 use of ocular or systemic corticosteroids, 29 and antineoplastic drugs. 30 Increased episcleral venous pressure due to conditions such as carotid-cavernous sinus fistulas may cause OAG. 31
Angle-closure glaucomas are rapidly progressive ocular neuropathies characterized by the occlusion of at least 270° of the iridocorneal angle. 3 Angle-closure glaucomas are only one-third as common as OAGs; however, they are responsible for approximately 50% of all glaucoma-induced blindness. Primary ACG, which arises from pupillary block (appositional closure of the iridocorneal angle that results from an increasing pressure differential between the anterior and posterior chambers of the eye 32 ) or plateau iris (an anteriorly positioned ciliary body that causes contact between the iris and TM with resultant angle crowding 33 ), has a global prevalence of 0.6%. 3 , 34 , 35 Primary ACG occurs most frequently in women, Asians, people with hypermetropic (short) eyes and people with shallow anterior chambers. 34 Affected patients require urgent treatment (usually laser iridotomy) to reverse obstruction of the angle. 34
Several secondary types of ACG are seen. Neovascular glaucoma, new blood vessels that occlude the angle, may develop from central retinal vein occlusion or diabetic retinopathy and generally carries a poor visual prognosis. 1 , 36 Phacomorphic glaucoma involves angle-closure because of lens intumescence (advanced cataract), and cataract removal typically leads to good visual recovery. 37 Angle-closure may be caused by corneal endothelium abnormalities (eg, iridocorneal endothelium syndromes) 38 or large iris or ciliary body masses. 39 Several medications, including anticholinergics, may precipitate ACG in eyes with preexisting narrow angles. 1 , 40
Differentiating between OAG and ACG is usually done via gonioscopic examination with slit lamp viewing. 41 Gonioscopy has long been the gold standard for visualizing the anterior chamber angle (ACA); however, challenges, including lens-eye contact, lack of objective measurements, a steep learning curve, and inconsistent interpretations between physicians, exist. 41 , 42 Advanced ACA imaging techniques including swept-source optical coherence tomography (OCT), goniophotography systems, and deep learning algorithms have been developed to overcome the limitations of gonioscopy. 43
Examination
Approximately 50% of individuals in the resource-limited countries are unaware that they have glaucoma, underscoring the importance of patient awareness education in diagnosis and management. 3 , 44 , 45 The diagnosis of glaucoma involves risk assessment, measurement of visual acuity, IOP, and corneal thickness, OCT imaging of the retinal nerve fiber layer (RNFL) and ONH, and visual field testing. Because most patients with glaucoma are asymptomatic for years, it is recommended that those with risk factors (advanced age, family history, non-White race, high IOP, and steroid use) be referred to an eye care provider for a glaucoma assessment. 3 , 4 , 5
Intraocular pressure needs to be monitored regularly in patients at a high risk of developing glaucoma. It is commonly measured using rebound tonometry (iCare ic100; iCare) or the “gold standard” Goldmann applanation tonometry. The iCare tonometer measures IOP-dependent rebound velocity after brief corneal contact, whereas Goldmann applanation tonometry measures the force required to flatten a 3.06-mm diameter segment of the cornea. 46 Agreement in measurements is good between the 2 devices; however, the reliability of the iCare decreases at higher IOPs and with thicker central corneas. 47 , 48 , 49 Normal IOP ranges from 11 to 21 mm Hg 50 ; however, IOP should be evaluated with consideration of optic nerve defects and/or high central cornea values. 51 Up to 50% of glaucomatous eyes have normal IOP measurements, 3 , 52 which emphasizes the importance of performing additional diagnostic imaging when indicated.
Making the diagnosis of glaucoma, particularly at an early stage, can be difficult because there is no uniform standard for diagnosis. 3 Structural changes of early glaucoma can be seen with OCT imaging of the optic nerve and macula, and functional changes in advanced glaucoma can be detected with visual field testing. Normal appearances of the ONH, RNFL, and visual field are shown in Figure 2 A, C, and E, respectively. All glaucomas are defined by ONH degeneration with disc excavation ( Figure 2 B) and RNFL thinning ( Figure 2 D). 53 Optic nerve head damage is characterized by thinning of the neuroretinal rim, usually in the superior and inferior quadrants, although the remainder of the ONH may remain pink with a normal neuroretinal rim. 3 , 53 Glaucomatous damage leads to retinal ganglion cell apoptosis, which can be seen as thinning between the internal limiting membrane and ganglion cell layer on OCT. 53 As glaucoma progresses, ONH and RNFL abnormalities cause visual field defects ( Figure 2 F). Visual field defects are often not observed in the early stages of glaucoma because peripheral vision and Snellen visual acuity are preserved until RNFL damage reaches an advanced stage. 51

Comparison of optic nerve head (ONH), retinal nerve fiber layer (RNFL), and visual fields in normal and glaucomatous eyes. A, Normal ONH with round, elevated ONH and a small central cup. B, Glaucomatous ONH with excavation and thinning of neuroretinal rim. C, Optical coherence tomography (OCT) examination shows normal RNFL thickness. D, OCT examination shows RNFL thinning in glaucomatous eyes. E, A full field in both eyes is shown. F, Abnormal visual field results in glaucomatous eyes are shown. The right eye field shows a superior altitudinal defect, moderate inferior arcuate defects, and a nasal step. The left eye field shows a superior paracentral defect with nasal step that splits fixation, an early inferior arcuate scotoma, and nasal step.
A general correlation between OCT imaging and visual field examination can be observed; however, there is no widely accepted method for comparing the two, 54 and diagnosing glaucoma is ultimately up to the discretion of the physician. Once glaucoma has been diagnosed, its severity must be categorized—typically as mild, moderate, or severe. Because all glaucoma types present with structural damage, most classification systems grade severity on the basis of functional visual field abnormalities. Most recently (2015), the International Classification of Diseases, Tenth Revision, released a grading system that associates mild glaucoma with a general absence of visual field defects, moderate glaucoma with visual field abnormalities in 1 hemifield (but outside 5° of fixation), and severe glaucoma with abnormalities in both hemifields and visual field loss within 5° of fixation. 55
Management of Glaucoma
Medical therapy.
Guidelines from the American Academy of Ophthalmology Preferred Practice Pattern (2020) state that an initial IOP reduction of 20%-30% is a suitable goal to slow disease progression, even in eyes with normal tension glaucoma. 56 The IOP must be carefully monitored during each follow-up visit, and the IOP control goal should be lowered further if progression continues. 56
Intraocular pressure–lowering medications have been the first-line therapy for most patients with glaucoma for several decades ( Table 2 ). Pharmacotherapy for glaucoma has evolved significantly over the past several decades with the introduction of topical carbonic anhydrase inhibitors (CAIs), beta blockers, prostaglandin analogs, and alpha agonists. 57 These medications have greater effectiveness and more favorable safety profiles than the older topical (pilocarpine) and systemic (oral CAIs) treatments. 57 In accordance with the generally accepted pharmacotherapy principles, the desired IOP range should be achieved with the fewest medications and least adverse effects. 3 Because of their tendency to induce glaucoma, ocular and systemic corticosteroids should be administered with caution in at-risk patients. 29
Table 2
US Food and Drug Administration–Approved Medications Used for the Treatment of Glaucoma
| Class | Medications | Adverse effects | Contraindications |
|---|---|---|---|
| Prostaglandin analogs | |||
| Cholinergic agonists | |||
| Carbonic anhydrase inhibitors | First generation (systemic): | First generation (systemic): | |
| Beta adrenergic antagonists | Nonselective: | ||
| Αlpha adrenergic agonists | |||
| Rho kinase inhibitors | |||
| Hyperosmotic agents |
Prostaglandin analogs (PGAs) are the most commonly used medications for the treatment of OAG and ocular hypertension. Prostaglandin analogs compensate for decreased TM outflow by increasing outflow through the uveoscleral pathway, 58 where aqueous humor moves through the ciliary muscle into the supraciliary and suprachoroidal spaces. 59 Prostaglandin analogs are administered once daily, are well tolerated, and have limited systemic adverse effects. 3 , 58 The main ocular adverse effects are eyelash growth, iris pigmentation, and uveitis. 56 Because most PGAs do not target the primary outflow pathway (TM), concerns have been raised about their long-term efficacy. 57 The recently approved latanoprostene bunod 0.024% may target the TM rather than the uveoscleral pathway, 57 , 60 and compared with timolol 0.5% over 3 months of follow-up, it has superior IOP-lowering efficacy and a comparable safety profile. 57 , 61 , 62 Prostaglandin analogs are a significant improvement over cholinergic agonists (such as pilocarpine), which induce miosis and increase conventional outflow by decreasing outflow resistance. 63 Pilocarpine, a mainstay of glaucoma treatment in the 1970s and 1980s, needed to be administered 4 times per day, a difficult regimen to maintain, which contributed to its being supplanted by beta blockers and PGAs. 3
Both CAIs and beta blockers lower the IOP by targeting the aqueous humor production in the ciliary body. After topical administration, CAIs penetrate the cornea and reach the ciliary body epithelium, where they reduce the production of bicarbonate ions. 64 The CAIs (dorzolamide 2% and brinzolamide 1%) are administered 2 or 3 times daily, 64 but they are generally less effective than PGAs and beta blockers, which limits their use as first-line therapy. Systemic CAIs (methazolamide and acetazolamide) are highly effective, which makes them useful in the treatment of ACG; however, their use is limited by their high incidence of adverse effects that cause 50% of patients to become intolerant after 1 month.
Beta adrenergic antagonists (beta blockers) block the sympathetic nerve endings in the ciliary body epithelium, which decreases the production of aqueous. 65 Beta blockers may be nonselective or cardioselective (β1 selective), the latter of which is well tolerated in patients with asthma and chronic obstructive pulmonary disease. 65 The advantages of beta blockers include their relatively low cost and once-daily administration. 3 , 5 Topically administered beta blockers enter the venous circulation but escape the first-pass metabolism in the liver, which predisposes the patient to pulmonary (bronchial constriction) and cardiac (arrythmias) disturbances. 5 , 66 Systemic absorption can be lessened by eyelid closure or gentle punctal occlusion for 2 minutes after topical administration. 3
Topical alpha-adrenergic agonists (brimonidine and iopidine) reduce the IOP by decreasing the aqueous humor production and increasing the outflow. 3 They are administered 2 or 3 times daily and are usually used as second-line agents in combination with other drugs. A retrospective study found that combination treatment (CAI+PGA) was more prevalent in everyday practice than alpha-2 agonists + PGA, suggesting that the administration of alpha-2 agonists may be accompanied by more adverse effects. 67
Rho kinase inhibitors are a recently introduced medication class that uses a combined mechanism of increasing the conventional outflow and decreasing the episcleral venous pressure. 68 Netarsudil 0.02%, a rho kinase inhibitor approved by the US Food and Drug Administration in 2017, has IOP-lowering efficacy comparable with that of timolol 0.5%, but with more frequent adverse effects. 59 , 69 , 70
Pharmacotherapy is an effective short-term treatment strategy; however, limitations to long-term use include cost, adverse effects, and failure to reach the target IOP. Nonadherence to the administration schedule is another significant issue because fewer than half of the patients with glaucoma regularly use antiglaucoma medications as prescribed after 1 year. 5 , 71
Laser Therapy
When pharmacotherapy fails to achieve the target IOP and prevent vision loss, laser and surgical procedures are indicated. Laser procedures effectively lower the IOP and minimize the long-term costs that are associated with the long-term use of multiple pressure-lowering medications. 5 A variety of laser procedures can be performed in glaucomatous eyes, with the procedure of choice depending on the etiology of the disease ( Table 3 ).
Table 3
Laser Procedures for the Treatment of Glaucoma
| Laser procedure | Preferred use | Pros | Cons |
|---|---|---|---|
| Laser trabeculoplasty | |||
| Excimer laser trabeculostomy | |||
| Laser peripheral iridotomy | |||
| Laser peripheral iridoplasty | |||
| Cyclodestructive procedures |
IOP, intraocular pressure; LPI, laser peripheral iridotomy; TM, trabecular meshwork.
Laser trabeculoplasty and ab-interno excimer trabeculostomy (Glautec AG) are both indicated for OAG that is refractory to pharmacotherapy. Laser trabeculoplasty—multiple spots of thermal laser applied directly to the TM—induces favorable structural changes that increase the aqueous humor outflow. 72 Argon laser trabeculoplasty, developed in 1979, uses a with a blue-green continuous-wave laser (488 and 514 nm) to disrupt the TM, whereas selective laser trabeculoplasty (SLT), developed in 1995, uses low energy, brief duration, large spots from a green, frequency-doubled laser to target melanin-containing cells and spare the TM tissue. 73 Selective laser trabeculoplasty has largely supplanted argon laser trabeculoplasty because of its favorable safety profile, comparable IOP-lowering efficacy, and ability for repeated treatment applications. 74 More recently introduced laser trabeculoplasty procedures include titanium-sapphire laser trabeculoplasty and pattern scanning trabeculoplasty. Limited short-term data suggest that both the procedures have efficacy and safety profiles similar to that of SLT. 74 Laser trabeculoplasty procedures are generally preferred over operations because they are less invasive and possess better safety profiles. 3 , 74 Ab-interno excimer trabeculostomy is a MIGS similar to laser trabeculoplasty that uses a 308-nm XeCl excimer laser to create microperforations in the TM and inner wall of the Schlemm canal. 75 Excimer trabeculostomy has a comparable safety profile and IOP-lowering efficacy similar to SLT over 2 years. 75
Patients with ACG require different laser procedures from those with OAG. A laser peripheral iridotomy creates a hole in the peripheral iris and is often performed to eliminate pupillary block, 76 whereas a laser peripheral iridoplasty uses low-power laser burns to relieve appositional angle closure (by shrinking the peripheral iris) in cases where laser peripheral iridotomy is ineffective. 77 When combined, both treatments have been shown to be safe and effective in lowering the IOP in eyes with acute primary ACG refractory to pharmacotherapy. 78 For eyes refractory to all other medical, surgical, and laser therapies, a series of cyclodestructive procedures that damage the ciliary body epithelium and decrease the IOP by reducing the aqueous humor secretion may be the final treatment option. 79 These procedures consist of endoscopic cytophotocoagulation (Endo Optiks), continuous-wave diode laser (IRIDEX Corp), or the newest alternative, MicroPulse transscleral laser therapy (IRIDEX Corp), which selectively targets the pigmented tissue of the ciliary body epithelium. 79 Cyclodestructive procedures are also useful for the secondary forms of glaucoma, such as uveitic, traumatic, or neovascular glaucoma; however, these procedures come have considerable risks and are particularly difficult to titrate. 79
Surgical Treatment
Operations are usually performed when medical and laser treatments have failed to achieve adequate IOP reduction. Surgical options consist of the traditional, bleb-based IOP-lowering operations (trabeculectomy and tube shunt implantation) and the newer, conjunctiva-sparing MIGSs ( Table 4 ). Bleb-based operations can effectively lower IOP; however, they may develop bleb-related complications and may have high reoperation rates. As a result, the current role of traditional procedures in the era of evolving MIGSs is unclear. Surgeons’ perspectives are changing 80 ; a recent practice preferences survey from the American Glaucoma Society (2017) found that trabeculectomy has fallen out of favor, with tube shunt implantation reported as the preferred incisional surgical treatment in 7 of 8 surgical centers. 81 When prospective MIGS trials are completed, the pendulum may swing in favor of MIGSs. 80
Table 4
Surgical Procedures for the Treatment of Glaucoma a
| Procedure | Type | Pros | Cons |
|---|---|---|---|
| Trabeculectomy | |||
| Ex-PRESS mini shunt operation | |||
| Valved drainage implants | |||
| Nonvalved drainage implants | |||
| Trabecular bypass | |||
| Canaloplasty | |||
| Ab-interno trabeculotomy; goniotomy | |||
| Trabeculotomy/viscodilation | |||
| Goniotomy/viscodilation | |||
| Ab-interno subconjunctival implant | |||
| Ab-interno suprachoroidal implant |
Trabecular Outflow Resistance
The juxtacanalicular tissue within the TM is the primary source of outflow resistance in eyes with POAG, with the inner wall of the Schlemm canal serving as an additional line of resistance. 82 , 83 , 84 To improve the aqueous outflow and lower the IOP, surgeons bypass the TM by directing the aqueous flow directly into the Schlemm canal or by rerouting the fluid from the anterior chamber into the subconjunctival space.
Traditional Incisional Operations
Trabeculectomy—the “gold standard” surgical glaucoma procedure for several decades—is the creation of a partial thickness scleral flap with excision of a segment of TM to create an alternate drainage route from the anterior chamber to the subconjunctival space. 85 , 86 Trabeculectomy can produce outstanding IOP control, particularly in eyes where an IOP near the low teens is targeted to slow glaucoma progression. 87 , 88 Trabeculectomy may be performed together with cataract extraction (CE) and/or administration of mitomycin C (MMC) on the surface of the sclera to prevent postoperative conjunctival fibrosis. 89 Trab-MMC alone, trab-MMC+CE, and trab-MMC in pseudophakic eyes were found to produce comparable IOP reductions and success rates after 5 years 90 ; however, other studies have found lower success rates with trab-MMC in pseudophakic eyes, probably because of postoperative inflammation after CE. 80 , 91
Tube shunt implantation, an alternative to trabeculectomy, has gained popularity in recent years. The implantation of tube shunts, often referred to as glaucoma drainage devices (GDDs), creates a permanent sclerostomy to drain the aqueous humor into the subconjunctival space. 92 The advantages of GDDs over trabeculectomy include decreased conjunctival scarring (by diverting aqueous drainage to the equatorial region of the eye and away from the limbus) and the formation of a permanent bleb (plate tube). 92 Most GDD designs are modeled after the early Molteno implant 93 and may be valved (promotes unidirectional flow) or nonvalved (passive-acting). 92 The Ahmed Baerveldt Comparison and Ahmed Versus Baerveldt studies compared the safety and efficacy of the valveless Baerveldt 350-mm 2 GDD (Johnson & Johnson) to that of the valved Ahmed-FP7 GDD (New World Medical Inc). Both devices were effective in reducing the IOP and the need for IOP-lowering medications, although a favorable IOP decrease, medication burden reduction, and safety profile (but with a higher incidence of hypotony) were seen with the valveless Baerveldt 350-mm 2 GDD at 5 years. 94 Recent advancements in valveless GDD operation include the development of the Ahmed ClearPath GDD (New World Medical Inc) and PAUL glaucoma implant (PGI; Advanced Ophthalmic Innovations). The Ahmed ClearPath GDD has several unique design features, such as a flexible, low-lying plate with anterior suture points to increase the ease of implantation, and a prethreaded 4-0 polypropylene ripcord to mitigate the risk of hypotony that has been reported in other GDD studies. 95 The PGI GDD has a smaller plate that occupies less space in the ACA and a relatively large endplate surface area through which the aqueous humor can be absorbed. 96 Early outcome data with the Ahmed ClearPath GDD and PGI found mean IOP reductions of 43% 97 and 51.6%, 96 at 6 months, respectively.
Both trabeculectomy and GDD implantation are effective treatment options for refractory glaucoma—eyes with poor results after both pharmacotherapy and laser. A 5-year comparison of trabeculectomy and tube shunt operation found that both techniques effectively lower the IOP (trabeculectomy: 49.5%; tube: 41.4%), with the tube group having a better safety profile. 97 In surgically naïve eyes with refractory glaucoma, the Primary Tube vs Trabeculectomy study found trabeculectomy to be superior, 98 whereas the Tube vs Trabeculectomy study reported similar outcomes in both groups at 5 years postoperatively in eyes that were not surgically naïve; however, eyes in the tube group had lower failure and reoperation rates. 17 , 97 Frequent complications within the early postoperative period included choroidal effusion (Tube, 14%; Trab, 13%) and shallow anterior chamber (Tube, 10%; Trab, 10%), and late postoperative complications included persistent corneal edema (Tube, 16%; Trab, 9%) and bleb encapsulation (Tube, 2%; Trab, 6%). 17 Many of the eyes needed postoperative interventions (Tube: 25%, Trab: 70%). 17 Craven et al 16 estimated that 25% of patients treated with trabeculectomy or a tube shunt needed additional interventions to address surgical failure.
Minimally Invasive Glaucoma Surgeries
The potential complications and surgical failures seen with traditional incisional operations speak to the need for better procedures for mild-to-moderate glaucoma that are minimally invasive yet durable. This has led to the introduction of MIGSs, which have revolutionized glaucoma care over the past decade. This group of novel procedures may sufficiently lower the IOP to delay or minimize the need for traditional incisional procedures, 82 and they are more suitable for patients with mild-to-moderate glaucoma. Minimally invasive glaucoma surgeries can be performed together with cataract operation, which makes them a valuable option for glaucomatous eyes with advanced cataracts (from aging, phacomorphic glaucoma, traumatic glaucoma, etc). Unlike the traditional filtration procedures, MIGSs are relatively simple to perform because they require surgical skills similar to those required for modern-day cataract surgery, 99 and they can be performed by cataract surgeons who are not glaucoma fellowship trained. Minimally invasive glaucoma surgeries have favorable safety profiles and are less invasive than traditional incisional operations. 100
One of the management challenges with performing MIGSs lies in whether to bypass or enhance the conventional aqueous outflow 101 because the currently available MIGS devices target 1 of the 3 pressure-lowering mechanisms: (1) the trabecular outflow pathway, referring to “angle-based” MIGSs that reroute the aqueous flow toward the Schlemm canal; (2) the subconjunctival space, referring to MIGSs that create a drainage pathway, diverting the aqueous humor to the subconjunctival space; (3) the suprachoroidal space, referring to MIGSs that increase the uveoscleral pathway outflow and divert the aqueous flow toward the suprachoroidal space. 100
MIGSs Targeting the Trabecular Outflow Pathway
Approximately 50%-75% of the outflow resistance lies within the TM and the inner wall of the Schlemm canal, whereas the remainder resides within the Schlemm canal and its distal collector channels. 102 , 103 , 104 , 105 This identifies the conventional outflow pathway as an attractive first target for the treatment of glaucoma. Angle-based MIGSs take advantage of the lower resistance within the Schlemm canal and divert the aqueous flow to the canal, thereby bypassing most of the outflow resistance. Despite this, however, a significant proportion of outflow resistance remains, thereby making these procedures unsuitable for patients with severe glaucoma who require significant IOP reduction. 80 Minimally invasive glaucoma surgeries that target the trabecular outflow pathway fall within the categories of trabecular bypass implant, ab-interno canaloplasty, ab-interno trabeculotomy (AIT), goniotomy, and the more recently introduced combined goniotomy/viscodilation and trabeculotomy/viscodilation procedures.
The iStent (Glaukos Corporation), the first trabecular bypass implant, has produced excellent results when implanted into glaucomatous eyes that are well-controlled on 1 IOP-lowering medication. 80 Additional IOP lowering is observed when placing more than 1 stent, which led to the development of the iStent inject and iStent inject W. 100 A study comparing the early outcomes of the iStent and iStent inject reported favorable IOP (iStent, 4.3%; iStent inject, 19.1%) and medication reduction results (iStent, 72.2%; iStent inject, 94.1%) in the iStent inject group at 12 months, with a similar safety profile observed in both the groups. 106 Ab-interno canaloplasty is typically performed with the iTrack microcatheter (Nova Eye Medical), and a retrospective comparison with ab-externo canaloplasty (iTrack with a 9-0 prolene tensioning suture) found comparable safety and efficacy. 107 Ab-interno trabeculotomy and goniotomy procedures bring the anterior chamber, Schlemm canal, and distal collector channels into direct communication through the disruption or partial excision of the TM. 108 The Trabectome (Neomedix), a long-standing AIT procedure, uses electrocauterization to ablate the TM and has been documented to safely and effectively reduce the IOP. 108 Recent advancements in excisional goniotomy include the Kahook Dual Blade (KDB; New World Medical) and KDB Glide (New World Medical) devices. Although limited data on KDB Glide exist within the literature, several studies of KDB have shown that it has a favorable safety profile and similar effectiveness to AIT procedures. 109 , 110
Angle-based MIGS procedures are easy to perform and have favorable safety profiles, but compared with traditional trabeculectomy, they have more limited abilities to lower IOP. 101 , 111 Distal outflow (collector channels and episcleral veins), which is often overlooked in the treatment of glaucoma, may play a pivotal role in IOP control and is unaffected by canalicular-based MIGS procedures. Studies with bovine and monkey eyes have found that collector channels may alter the pressure distribution within the Schlemm canal, suggesting that the aqueous outflow may depend on the location of these distal elements. 84 , 102 , 112 Resistance within the Schlemm canal and the collector channels appears to limit the outflow increase of trabecular bypass procedures to 13%-26% and IOP reduction to the mid-teens, but a greater pressure decrease is expected if a moderate dilation of the Schlemm canal and the collector channels is achieved. 84 , 113 , 114 Goniotomy and trabeculotomy may be performed concurrently with the implantation of an ophthalmic viscosurgical device (STREAMLINE Surgical Systems, New World Medical; OMNI360 Surgical Systems, Sight Sciences) to the Schlemm canal to reduce the distal outflow resistance and promote further IOP reduction. Interim analyses of the STREAMLINE and OMNI trials have shown effective, sustained IOP reductions and meaningful medication reductions at 6 and 12 months, respectively. 115 , 116
MIGSs Targeting the Subconjunctival Space
Minimally invasive glaucoma surgeries devices within this category work similarly to trabeculectomy by diverting the aqueous humor flow directly into the subconjunctival space. 100 The main disadvantage of this strategy is the potential for subconjunctival fibrosis, which for trabeculectomy may be prevented by the intraoperative application of MMC. 100 Subconjunctival MIGS devices, which are designed based on the Hagen-Poiseuille equation, include the ab-internally implanted XEN45 gel stent (Allergan) and the ab-externally implanted PRESERFLO microshunt (Santen). Both devices produce comparable safety profiles, IOP reductions, and overall surgical success at 2 years. 117 The analysis of both implantation approaches with an experimental microfluidic system found higher outflow resistance and less predictable bleb formation with ab-interno implantation. This may affect the long-term IOP control and could direct the development of future subconjunctival-based MIGS devices. 118
MIGSs Targeting the Suprachoroidal Space
The third category of MIGSs aims to increase the uveoscleral outflow. 100 The uveoscleral pathway is not limited by the pressure “floor” formed by episcleral venous pressure; thus, diverting the aqueous humor into the suprachoroidal space could have a greater lower IOP capacity. 119 Unfortunately, current studies have yet to realize such results. After the recall of CyPass (Alcon) in 2018 because of corneal endothelial cell loss from malpositioned devices, most suprachoroidal MIGSs are still under investigation. 119 A review of recent studies indicates favorable safety profiles and effective short-term IOP reductions to the mid-teens with the iStent SUPRA (Glaukos Corporation), STARflo (iSTAR Medical), and gold implant (SOLX, Inc). Longer follow-ups and more robust trial designs are still required for the US Food and Drug Administration approval of suprachoroidal MIGSs, 120 and long-term efficacy may be limited by fibroblast migration and proliferation. 121
The pathogenesis of glaucoma is multifactorial and incompletely understood, and diagnosis methods and management strategies are constantly being improved. Treatment outcomes, safety profiles, and recovery times have improved with the introduction of MIGSs. Future work should aim to develop MIGS devices with greater IOP-lowering capabilities than traditional incisional operations.
Potential Competing Interests
The authors report no competing interests.
Acknowledgments
The authors acknowledge Jason S. Calhoun, COA, for the ultrasound biomicroscopy image ( Figure 1 ).
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 29 September 2021
- Correction 06 October 2021
Reversing blindness with stem cells
- Neil Savage 0
Neil Savage is a freelance writer in Lowell, Massachusetts.
You can also search for this author in PubMed Google Scholar
Nick Kharufeh celebrated US Independence Day last year with his family, by setting off fireworks outside his aunt’s home in Rialto, California. A large rocket, instead of flying skywards, hit the ground and exploded, shooting a burning fragment into his face and immediately blinding him in his left eye. At the hospital, doctors told him his eyelid, cornea and lens had been burnt beyond saving, and that he was not a good candidate for a corneal transplant. With that news, the 23-year-old began to face the prospect of living with just one eye, and tried to accept that his dream of becoming a commercial pilot was probably over.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Nature 597 , S24-S26 (2021)
doi: https://doi.org/10.1038/d41586-021-02629-w
This article is part of Nature Outlook: Stem cells , an editorially independent supplement produced with the financial support of third parties. About this content .
Updates & Corrections
Correction 06 October 2021 : An earlier version of this Outlook article misspelt Kharufeh’s name throughout.
Rama, P. et al. N. Engl. J. Med. 363 , 147–155 (2010).
Article PubMed Google Scholar
Hayashi, R. et al. Nature 531 , 376–380 (2016).
Gouveia, R. M. & Connon, C. J. Trans. Vis. Sci. Tech. 9 , 5 (2020).
Article Google Scholar
Isaacson, A., Swioklo, S. & Connon, C. J. Exp. Eye Res. 173 , 188–193 (2018).
Oswald, J., Kegeles, E., Minelli, T., Volchkov, P. & Baranov, P. Mol. Ther. 21 , 180–198 (2021).
Download references
Related Articles

- Regeneration
- Biotechnology
- Therapeutics
Stem cells tightly regulate dead cell clearance to maintain tissue fitness
Article 21 AUG 24
Short-term post-fast refeeding enhances intestinal stemness via polyamines
Human organoids with an autologous tissue-resident immune compartment
Article 14 AUG 24
Acquisition of epithelial plasticity in human chronic liver disease
Article 22 MAY 24
Cells destroy donated mitochondria to build blood vessels
News & Views 01 MAY 24
Airway hillocks are injury-resistant reservoirs of unique plastic stem cells
Article 01 MAY 24
Five ways science is tackling the antibiotic resistance crisis
News Feature 13 AUG 24

Second brain implant by Elon Musk’s Neuralink: will it fare better than the first?
News 06 AUG 24
Suzhou Institute of Systems Medicine Seeking High-level Talents
Full Professor, Associate Professor, Assistant Professor
Suzhou, Jiangsu, China
Suzhou Institute of Systems Medicine (ISM)

Tenure Track Faculty Position - Division of Hematology
The Division of Hematology at Washington University in St. Louis invites applications from outstanding candidates with a Ph.D. and/or M.D. degree f...
Saint Louis, Missouri
Washingon University School of Medicine - Division of Hematology
Lecturer/Senior Lecturer at the Dyson School of Design Engineering
About the role: Do you want to change the world for the better, and do you believe this can be done through research and education? If so, grab you...
South Kensington, London (Greater) (GB)
Imperial College London (ICL)
Faculty Positions in Westlake University
Founded in 2018, Westlake University is a new type of non-profit research-oriented university in Hangzhou, China, supported by public a...
Hangzhou, Zhejiang, China
Westlake University
Call for Global Talents, Recruitment Information of Nankai University
Nankai University welcomes global outstanding talents to join for common development.
Tianjin, China
Nankai University
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Responsiveness to Selective Laser Trabeculoplasty in Open-Angle Glaucoma and Ocular Hypertension
Collaborators.
- LiGHT China Trial Study Group : Mingkai Lin , Xing Liu , Xiulan Zhang , Jian Ge , Jingjing Huang , Yunlan Ling , Yimin Zhong , Chengguo Zuo , Jiangang Xu , Hui Xiao , Yixiang Huang , Yuantao Hao , Mingjie Deng , Yiming Ye , Zongyi Zhan , Shitong Huang , Yunzhen Wang , Yunzhi Xu
Affiliations
- 1 State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangdong Provincial Key Laboratory of Ophthalmology and Visual Science, Guangdong Provincial Clinical Research Center for Ocular Diseases, Guangzhou, China.
- 2 National Institute for Health and Care Research Biomedical Research Centre at Moorfields Eye Hospital National Health Service Foundation Trust and University College London Institute of Ophthalmology, London, United Kingdom.
- PMID: 39172470
- PMCID: PMC11342221 (available on 2025-08-22 )
- DOI: 10.1001/jamaophthalmol.2024.3133
Importance: Selective laser trabeculoplasty (SLT) is becoming the recommended first choice in the treatment of open-angle glaucoma (OAG). However, whether repeat SLT can be recommended regardless of initial response remains controversial.
Objective: To assess the potential of OAG and ocular hypertension (OHT) undergoing repeat laser to respond favorably to SLT, termed responsiveness to SLT.
Design, setting, and participants: This post hoc analysis of the Laser in Glaucoma and Ocular Hypertension Trial in China (LiGHT China) was conducted from March 2015 to April 2023 in Zhongshan Ophthalmic Center. Of 1376 newly diagnosed OAG and OHT eyes of 771 adults in the original trial, 180 eyes of 105 participants were included in the present study, which underwent initial and repeat SLT as primary treatments.
Exposures: Standard SLT was the primary treatment. Repeat SLT was the first choice of treatment escalation regardless of initial response. IOP reduction after SLT and the duration of effect were analyzed. The maximum reduction in IOP within 2 years after initial SLT and repeat SLT was used to identify potential nonresponsiveness.
Main outcomes and measures: IOP reduction 2 months after SLT.
Results: A total of 180 eyes from 105 Chinese participants (mean [SD] age, 45.6 [14.5] years; 58 [55.2%] male and 47 [44.8%] female) underwent repeat SLT. Initial SLT and repeat SLT were both associated with a reduction in IOP (mean, 4.5 mm Hg; 95% CI, 3.9 to 5.1; P < .001 and mean, 3.3 mm Hg; 95% CI, 2.7 to 3.8; P < .001, respectively). The mean (SD) IOP after repeat SLT was 15.8 (3.4) mm Hg, similar to 16.0 (4.0) mm Hg after initial SLT (difference, -0.4mm Hg; 95% CI, -1.0 to 0.3; P = .24). Duration of effect after repeat SLT was longer than after initial SLT (1043 days vs 419 days; hazard ratio, 0.38; 95% CI, 0.29 to 0.50; P < .001). IOP reduction after initial SLT was uncorrelated with that after repeat SLT, and 153 eyes (85.0%) responded favorably to SLT at least once. A subset of 27 eyes (15.0%) was identified as potentially nonresponsive and found distinctive with older age (mean [SD], 54.1 [12.5] years vs 44.2 [14.2] years; difference, 10.5 years; 95% CI, 2.9 to 18.1; P = .009), higher proportion of female participants (difference, 27.5%; 95% CI, 3.6 to 51.5; P = .03), and lower baseline IOP (difference, -3.2 mm Hg; 95% CI, -5.2 to -1.3; P = .001).
Conclusions and relevance: These post hoc analyses showed that most cases of OAG and OHT were highly responsive to SLT and support the consideration of repeat SLT regardless of initial response, while individuals who are nonresponsive to this treatment may have specific features.
PubMed Disclaimer
Conflict of interest statement
Conflict of Interest Disclosures: Dr Gazzard reported personal fees from Alcon Laboratories, personal AbbVie, Belkin, Elios, Lumenis, and Lumibird/Quantel/Ellex during the conduct of the study as well as personal fees from Genentech/Roche, Glaukos, Reichert, Ripple, Santen, Sight Sciences, Thea, and Vialase outside the submitted work. No other disclosures were reported.
- doi: 10.1001/jamaophthalmol.2024.3279
- Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390(10108):2183-2193. doi:10.1016/S0140-6736(17)31469-1 - DOI - PubMed
- Song P, Wang J, Bucan K, Theodoratou E, Rudan I, Chan KY. National and subnational prevalence and burden of glaucoma in China: a systematic analysis. J Glob Health. 2017;7(2):020705. doi:10.7189/jogh.07.020705 - DOI - PMC - PubMed
- American Academy of Ophthalmology . Primary open-angle glaucoma PPP 2020. Accessed July 24, 2024. https://www.aao.org/education/preferred-practice-pattern/primary-open-an...
- National Institute for Health and Care Excellence . Glaucoma: diagnosis and management. Accessed July 24, 2024. https://www.nice.org.uk/guidance/ng81
- European Glaucoma Society . Terminology and guidelines for glaucoma (5th edition). Accessed July 24, 2024. https://eugs.org/educational_materials/6 - PubMed
Related information
Linkout - more resources, full text sources.
- Silverchair Information Systems
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

- Conferences
- Publications
Impact of exercise on intraocular pressure in glaucoma patients
Rationale for delving into this topic is the recent studies that have reported that active and healthy living can reduce IOP.

(Image Credit: AdobeStock/Kzenon)

A new Spanish study found that exercise, especially aerobic exercise, may help modulate intraocular pressure (IOP) and may serve as a complementary therapy for patients with glaucoma , according to the lead author Daniel González-Devesa, PhD, from the Faculty of Physical Activity and Sports Sciences, Universidad de León, León, Spain.
Their rationale for delving into this topic is the recent studies that have reported that active and healthy living can reduce IOP. Many patients have consulted their doctors for lifestyle guidance based on this evidence. 2
Further, they pointed out, “Physical activity is an important but often overlooked factor that affects glaucoma progression, according to recent research. 3 However, the scientific community has yet to reach a consensus regarding the effects of exercise on glaucoma. Some theories suggest that an exercise-induced IOP elevation may lead to reduced ocular perfusion pressure, possibly causing mechanical or ischemic damage to the optic nerve head. 4 In contrast, other studies proposed that exercise can trigger a reduction in IOP levels, thus positively affecting ocular health. 5 ”
To increase the body of evidence regarding the impact of physical exercise on IOP, González-Devesa and colleagues conducted a literature search of English, Portuguese, or Spanish studies on the effect of exercise on IOP in glaucoma. They excluded case reports and yoga-based interventions. Of 1,001 identified records, 15 studies were independently evaluated. Of the 15 studies that were evaluated through the Mixed Methods Appraisal Tool scoring system, two quantitative randomized controlled studies scored 100% and 13 non-randomized studies averaged 84.62%, the investigators explained.
“Our findings indicated that both aerobic and resistance training led to an immediate IOP reduction post-exercise. In addition, exercise may serve as complementary therapy in glaucoma patients, potentially reducing glaucoma progression risk,” González-Devesa and colleagues reported.
Further, they offered the caveat that their findings were in large part obtained from single-session experiments and that the effects of longer-term exercise programs on IOP varied.
However, while they believe that their study underscores the potential benefit of exercise in IOP management, “the evidence remains inconclusive due to variations in study design, participant demographics, and exercise parameters. This lack of consistency in the research highlights the necessity for larger, standardized, and longer-term studies to robustly corroborate these preliminary findings,” they commented.
References:
González-devesa d, suárez-iglesias d, diz jc, et al. systematic review on the impact of exercise on intraocular pressure in glaucoma patients. int ophthalmol. 2024;44:351; https://doi.org/10.1007/s10792-024-03216-4, hecht i, achiron a, man v, burgansky-eliash z. modifiable factors in the management of glaucoma: a systematic review of current evidence. graefe arch clin exp ophthalmol. 2017;255:789–796. https://doi.org/10.1007/s00417-016-3518-4, olszewska h, kosny j, jurowski p, jegier a (2020) physical activity of patients with a primary open angle glaucoma. int j ophthalmol. 2020;13:1102–1108. https://doi.org/10.18240/ijo.2020.07.14, mcmonnies cw. intraocular pressure and glaucoma: is physical exercise beneficial or a risk j optom. 2016;9:139–147. https://doi.org/10.1016/j.optom.2015.12.001, kumar h, taneja s. commentary: exercise and intraocular pressure: friends or foes indian j ophthalmol. 2022;70:4236–4237. https://doi.org/10.4103/ijo.ijo_2258_22.

Researchers develop ultra-thin battery powered by saline for smart contact lenses

EyePod: Transforming the MIGS pipeline into clinical reality

The American Academy of Ophthalmology, Research to Prevent Blindness announce recipients of RPB/AAO Award for IRIS Registry Research

Modifying and optimizing a new era of glaucoma surgeries

Proactive glaucoma management: Tailoring treatment for enhanced patient outcomes

Study links statin use to higher risk of glaucoma
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

- Open access
- Published: 28 August 2024
Engineered sensor actuator modulator as aqueous humor outflow actuator for gene therapy of primary open-angle glaucoma
- Samarendra Mohanty ORCID: orcid.org/0000-0002-6533-383X 1 ,
- Subrata Batabyal 1 ,
- Chinenye Idigo 1 ,
- Darryl Narcisse 1 ,
- Sanghoon Kim 1 ,
- Houssam Al-Saad 1 ,
- Michael Carlson 1 ,
- Kissaou Tchedre 1 &
- Adnan Dibas 1
Journal of Translational Medicine volume 22 , Article number: 791 ( 2024 ) Cite this article
Metrics details
Glaucoma, a blinding eye disease with optic neuropathy, is usually associated with elevated intraocular pressure (IOP). The currently available pharmacological and surgical treatments for glaucoma have significant limitations and side effects, which include systemic reactions to medications, patient non-compliance, eye infections, surgical device failure, and damage to the eye. Here, we present Sensor-Actuator-Modulator (SAM), an engineered double mutant version of the bacterial stretch-activated mechanosensitive channel of large conductance (MscL) that directly senses tension in the membrane lipid bilayer of cells and in response, transiently opens its large nonspecific pore to release cytoplasmic fluid. The heterologously expressed mechanosensitive SAM channel acts as a tension-activated pressure release valve in trabeculocytes. In the trabecular meshwork (TM), SAM is activated by membrane stretch caused by elevated IOP. We have identified several SAM variants that are activated at physiologically relevant pressures. Using this barogenetic technology, we have demonstrated that SAM is functional in cultured TM cells, and successfully transduced in vivo in TM cells by use of AAV2/8. Further, it is effective in enhancing aqueous humor outflow facility leading to lowering the IOP in a mouse model of ocular hypertension.
One sentence summary
Autoregulation of intraocular pressure via expression of a mechanosensitive channel of large conductance in trabecular meshwork serves as a mutation-agnostic gene therapy for glaucoma.
Introduction
Cells of different organs in the body undergo a range of mechanical and osmotic pressures which change in various diseases including neurological, cardiovascular, ophthalmological, and renal diseases. Clogging of the outflow pathway in the eyes and brain leads to hypertension and related injury. Glaucoma is usually associated with elevated intraocular pressure (IOP). Vision loss due to glaucoma is the second leading cause of blindness [ 1 ]. The currently available pharmacological and surgical treatments for glaucoma have significant limitations and side effects, which include systemic reactions to medications, patient non-compliance, eye infections, surgical device failure, and damage to other structures of the eye [ 2 ]. Primary open-angle glaucoma (POAG) is common and is characterized by poor drainage of aqueous humor (AH) through the conventional outflow pathway. Previous studies have shown overexpression of fibrotic proteins [ 3 , 4 ] in glaucomatous trabecular meshwork (TM) is related to increased stiffness of TM along with modulation of TM cell behavior [ 5 , 6 ]. Increased extracellular matrix deposition and cytoskeletal rearrangements of cross-linked actin networks (CLANs) in the glaucomatous TM cause AH outflow resistance [ 3 , 7 ]. Continuous turnover of extracellular matrix (ECM) is required for the maintenance of IOP regulation. This proteinaceous build-up inhibits the translocation of aqueous humor through the TM leading to IOP elevation. Several TM ECM manipulations have been conducted to modulate the outflow facility. The approved drugs address the improvement of drainage through the conventional outflow pathway [ 2 , 8 ] by either reducing AH production (e.g. Beta-blockers [ 9 ]) or increasing uveoscleral outflow (e.g. prostaglandins) [ 8 ] or TM outflow (Rhopressa [ 10 ]).
Though more than a hundred genes have been identified to be associated with glaucoma [ 11 ], no single gene is known to account for more than a small fraction of the glaucoma population [ 12 ]. Further, since no common convergent pathway(s) have been identified at this point, classical mutation-specific gene replacement/editing approaches [ 13 ] would require many therapies to be developed to address various mutations one at a time. The development of a safe effective long-lasting single-dose therapeutic for the ubiquitous treatment of glaucoma would transform the field of ophthalmology. The putative mechanosensitive channel TRPV4 plays a regulatory role in the Ca 2+ -dependent cytoskeleton rearrangement signaling cascade and its inhibition increases aqueous humor outflow and decreases TM stiffness and IOP [ 7 , 14 ].
The proposed use of a more effective mechanosensitive Sensor-Actuator-Modulator (SAM) channel-based gene therapy is inspired by existing physiological concepts of large pore mechanosensitive channels of Large conductance (MscL) [ 15 , 16 , 17 ]. SAM is activated by any mechanical force that causes deformation of the lipid bilayer, such as stretch, pressure/suction, turgor pressure, and ultrasound. Cell contraction and/or relaxation can similarly change the shape of the cells within the TM and Schlemm’s canal to promote AH outflow. Although some drugs stimulate outflow in this manner, it may not be sufficient to lower the IOP. Here, we put forth a novel commission for the engineered mechanosensitive channel (SAM) as a virally delivered transgenic pressure modulator in the TM cells of glaucomatous eyes. In the TM, SAM is activated by membrane stretch caused by elevated IOP. Using this barogenetic technology, we have demonstrated that SAM is functional in cultured TM cells, and successfully transduced in vivo in TM cells by use of AAV2/8. Further, we demonstrate effective lowering of the IOP upon SAM transduction in TM in a mouse model of ocular hypertension.
Expression of SAM in transfected trabecular meshwork cells
To evaluate SAM expression, the SAM gene, encoding for 136 amino acids, was codon optimized for mammalian cell expression and C-terminally fused to mCherry (fluorescent reporter). The matrix Gla protein promoter (pMGP) was used to target TM cells [ 18 ]. The construct was cloned and confirmed by agarose electrophoresis. The human TM (transformed via SV40) cells were characterized to evaluate the upregulation of TM markers upon Dexamethasone (DEX) treatment. Supplementary Fig. 1 shows enhanced Myocilin, PAI-1, Collagen-IV, and Fibronectin immunostaining in DEX-treated TM cells as compared to the negative control. The Western blot confirmed the upregulation of Fibronectin in DEX-treated TM cells. The TM cells were transduced with AAV8 carried SAM-mCherry and protein expression was determined by confocal microscopy and Western blot. Bright-field image of SAM-mCherry transfected human TM cells is shown in Fig. 1 A. The viability of the SAM-transfected TM cells was confirmed by Calcein AM staining (Fig. 1 B). The fluorescence (mCherry-reporter) image of human TM cells expressing SAM is shown in Fig. 1 C. Further, an anti-SAM antibody was used to evaluate the protein expression and characterization. After transfection of SAM using lipofection or AAV8 (different titers) transduction in cultured human TM cells, the proteins were isolated and subjected to immunoprecipitation using anti-SAM antibody. Figure 1 D shows the chemiluminescence Western blot of immunoprecipitated SAM trimers extracted from the human TM cells.

Expression of SAM-mCherry in transfected trabecular meshwork (TM) cells and kinetics of inward current in SAM-expressing TM cells under hypo-osmotic shock. (A) Bright Field image of human TM (Transformed via SV40) cells, (B) Calcein image of human TM cells after SAM transfection showing live cells, (C) Fluorescence (mCherry-reporter) image of TM cells expressing SAM. Scale bar: 1 mm. (D) Western blot confirming SAM (predominantly trimer, estimated MW of trimer = ~ 126 kDa) expression in human TM cells, also shown is non-transfected -ve control. (E) Fluorescence (mCherry-reporter) image of HEK cells expressing SAM. Scale bar: 100 μm. (F) The inward current profile of a SAM-expressing in HEK 293 (Top: red trace) and rat TM (Bottom, green trace) cell subjected to a hypotonic environment (addition of 20% v/v water). Insets show the respective current trace for non-transfected HEK (top panel: black trace) and TM (bottom panel: black trace) cells (-ve control). (G) Comparison of SAM channel peak current and open dwell time in SAM-transfected TM and HEK cells along with the non-transfected cells (-ve control). N = 5. Av.± S.D. ** p < 0.01 (Mann Whitney test). (H) The inward current profile of SAM-expressing cells subjected to -15 mmHg (black trace) and − 30 mmHg (red trace). The inset (blue trace) shows the current profile of non-transfected cells subjected to -30 mmHg. (I) Quantitative comparison of inward current for 2 different hold-pressures as compared to no pressure control in SAM-transfected cell, and -ve control (non-transfected) cell. N = 4, Av.± S.D., *** p < 0.001 (Mann Whitney test). (J) SAM-channel activity (blue trace) to sharp (~ 50 ms) pressure changes (up to pressure application: red section of trace)
Functioning of SAM channels in TM cells is different from that in other mammalian cells
To evaluate the functioning of SAM in different mammalian cells, SAM-transfected cells (i.e., HEK and TM, Fig. 1 E) and non-transfected (-ve control) cells were subjected to whole cell patch clamp electrophysiology. Channel activity was induced by subjecting the patched cell to a hypo-osmotic shock by adding 20% v/v water (Fig. 1 F). The comparison of SAM channel peak current and open dwell time (duration of channel opening) in TM and HEK cells showed that the host membrane greatly affects channel properties (Fig. 1 G). Significantly higher SAM-peak current was observed in TM cells as compared to HEK cells. However, no significant changes in open dwell time in TM and HEK cells were observed (Fig. 1 G).
Next, automated pressure-clamp electrophysiology was used to measure the effect of varied pressure on SAM-expressing mammalian cells. The cultured cells transfected with SAM were transferred to a patch clamp chamber in which negative pressure was created by use of a suction pump to exert a definite pressure on the cells. Inward current profiles of SAM-expressing cells subjected to -15 mmHg and − 30 mmHg are shown in Fig. 1 H. Figure 1 I shows a quantitative comparison of inward current for 2 different hold-pressures as compared to no pressure control in SAM-transfected cell and non-transfected (-ve control) cell. SAM-channel activity to sharp (~ 50 ms) pressure changes (Fig. 1 J) was higher than that during sustained elevated pressure conditions (Fig. 1 I).
Intracameral injection of AAV carried SAM-mCherry led to transduction of TM without apoptosis or eliciting immune response
To evaluate SAM expression in-vivo, intracameral injection of AAV2/8-pMGP-SAM-mCherry (vSAM: 6.75 × 10 10 vg/eye) was carried out in mice. Intracameral AAV-SAM-mCherry (vSAM) injection in mice led to SAM-mCherry (intrinsic) expression in the iridocorneal junction (Fig. 2 A, B). The DEX-only control group (Fig. 2 C, D) did not exhibit the characteristic expression. Figure 2 E shows a quantitative comparison of fluorescence intensity of mCherry-reporter signal in the TM for SAM-treated and -ve control eyes. To evaluate apoptosis of TM expressing SAM, immunofluorescence imaging of Caspase-3 in an anterior chamber flat mount was carried out. Cells in surrounding tissues, e.g., lens, corneal endothelium were not transduced by SAM as no expression of the reporter (mCherry) protein was observed (Fig. 2 F). Figure 2 F&G show the SAM-mCherry (red) expression and Caspase-3 staining (green) respectively, 10 weeks after vSAM treatment of DEX-mouse. No detectable apoptotic cell in vSAM-treated mice was observed (Fig. 2 G). Figure 2 H&I show immunostained images for mCherry (red) and Caspase-3 in wild-type PBS-injected (-ve control) mice. Confocal Images of iridocorneal regions in the axial sections of the eye transfected by vSAM are shown for: TM-marker Myocilin (Fig. 2 J), and SAM-mCherry-reporter (Fig. 2 K). Overlay of TM-marker and mCherry reporter shows an expression of SAM in TM (Fig. 2 L, M). Western blot of anterior chamber lysate from vSAM-injected mice confirms SAM expression (Fig. 2 N).

Intracameral injection of AAV carriedSAM-mCherry led to transduction of trabecular meshwork (TM) in-vivo. Representative fluorescence and Bright f ield images of f lat mount anterior segment showing ( A , B ) SAM-mCherry (intrinsic) expression in iridocorneal junction in mice 4 weeks post intracameral AAV-SAM-mCherry (vSAM) injection; ( C , (D) DEX-only control group, ( E ) The bar plot showing intensity of mCherry-reporter signal in the TM for SAM-treated and control (PBS) eyes. N = 5 , Av ± SD. ** p < 0.01 (Mann Whitney test). Fluorescence images of anterior chamber flat mount immunostained with mCherry (red, reporter for SAM) and Caspase-3 (green) for ( F - G ) DEX mouse, 10 weeks after vSAM treatment; ( H - I ) wild-type -ve control (PBS-injected) mouse. No detectable apoptotic cells in vSAM-treated mice. Confocal Images of iridocorneal regions in the axial sections of the eye transfected by vSAM: (J) Myocilin (TM-marker), (K) mCherry-reporter, and (L, M) Overlay. (N) Western blot confirming SAM (estimated MW = ~ 42 kDa) expression in mice anterior chamber lysate. High-resolution immunostained images of SAM-mCherry expression in TM in mice 24 weeks post intracameral AAV-SAM-mCherry (vSAM) injection: (O) DAPI, (P) mCherry (red, reporter for SAM), and (Q) overlay. (R) Pharmacokinetics of SAM using mCherry-reporter fluorescence intensity in the TM, N = 6 eyes, Av ± SEM. ** p < 0.01
Further, to understand the efficacy of SAM transduction in glaucoma-affected TM cells in vivo, we monitored mCherry reporter fluorescence in TM of IOP-elevated animal group. High-resolution immunostained images of SAM-mCherry expression in TM in mice 24 weeks post intracameral AAV-SAM-mCherry (vSAM) injection are shown in Fig. 2 O-Q. Figure 2 R shows the pharmacokinetics of SAM using mCherry-reporter fluorescence intensity in the TM. Stable expression of SAM was observed from 4 weeks of vSAM injection. To evaluate if vSAM injection leads to local immune response, the anterior chamber of SAM-injected mice was immunostained with Iba1 (a marker for microglia) and IFNg (an inflammatory cytokine). Supplementary Fig. 2 A-D show no immune response in vSAM-injected eyes as compared to -ve controls. Further, a quantitative comparison of IL-6 in plasma between baseline and after 1 and 10 weeks of vSAM transduction was carried out. As shown in Suppl. Figure 2 E, there was no significant change in systemic inflammatory response observed due to vSAM injection.
SAM lowers IOP in a dexamethasone-induced model of Glaucoma, mediated by outflow facility
The progressive IOP elevation in DEX-induced mice [ 20 ] was observed as compared to vehicle-applied mice (Fig. 3 B). The schematic of the experimental plan for DEX application (for IOP elevation), intracameral injection, IOP measurement, and end-of-study timeline is shown in Fig. 3 A. In Fig. 3 B, we show elevated IOP in mice (Arms 1–3) treated with continuous topical application of DEX as early as 1 week. In Group 2, eyes were subjected to intracameral vSAM injection just after baseline IOP measurements. In this Arm, the IOP continued to be elevated due to the DEX application. However, a significant IOP decrease was observed 3 weeks after intracameral injection of vSAM targeted to the TM. In Group 3, intracameral vSAM injection was carried out after observing IOP elevation (at 3 weeks). Immediate reduction in IOP (after vSAM injection) was observed, which can be attributed to the injection itself (though such reduction was not observed in Group 2, which may be due to the dominating effect of IOP elevation by DEX). Similar to Group 2, a statistically significant decrease in IOP as compared to the elevated IOP was observed in the vSAM-injected group in Group 3 (Fig. 3 B).
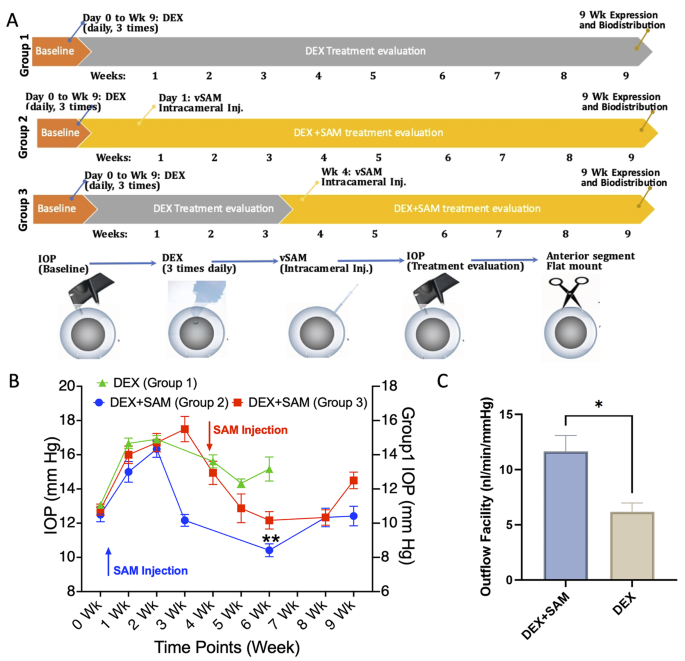
SAM lowers Intraocular Pressure (IOP) in a Dexamethasone-induced model of Glaucoma. (A) Schematic of DEX application, intracameral injection, IOP measurement, and end-of-study timeline. (B) Elevated IOP in mice (Groups 1 –3 ) treated with topical application of 0.1% DEX, 3 times daily. Group 1 (green): IOP e levat ion in mice treated with DEX. Group 2 (blue) and Group 3 (red): IOP in DEX-treated IOP-elevated mice decreases after intracameral injection of vSAM targeted to the TM. N = 8 eyes/Arm, Av ± SEM. ** p < 0.01. Arrows indicate vSAM injection time for Groups 2 and 3. Due to attrition, IOP measurements for DEX-control Group 1 are not available for 7–9 weeks. (C) Measured outflow in DEX-treated control vs. SAM-injected DEX-mice, Av ± SD, * p < 0.05
To determine changes in the aqueous outflow facility due to vSAM transduction of TM, aqueous humor hydrodynamics measurement [ 21 ] was conducted in anesthetized mice. The linear relationship of pressure versus flow rate in the anesthetized wild-type mouse eye can be seen in Suppl. Figure 3 D, leading to the determination of the outflow facility. Figure 3 C shows significantly higher measured outflow in SAM-injected DEX-mice as compared to DEX-treated control.
Intracameral injection of SAM protects the RGC as measured by visually evoked potential
To evaluate if intracameral injection of vSAM protects the RGC by lowering IOP, visually evoked potential (VEP) measurements were carried out in DEX-control and DEX + vSAM groups. VEP of DEX-mice and DEX-mice injected with vSAM at a light intensity of 7 × 10 13 photons cm -2 s -1 , 5 weeks after vehicle injection are shown in Fig. 4 A & B respectively. As shown in the figure, the VEP amplitude (~ 15 µV) of DEX-control mice is much lower than the VEP measured in vSAM-treated DEX-mice (~ 30 µV). The quantitative comparison shows significantly higher VEP amplitude in the vSAM-injected group as compared to DEX-only mice, 5 weeks after injection (Fig. 4 C). The mean difference of VEP amplitude between non-transfected and vSAM-injected DEX-mice is shown as Gardner-Altman estimation plot (Fig. 4 D).

SAM-induced lowering of IOP was neuroprotective to adult mice RGCs under Glaucomatous condition. Visually evoked potential (VEP) in DEX-mice with and without intracameral injection of AAV-SAM-mCherry (vSAM): (A) Representative VEP of DEX-mice, 5 weeks after vehicle injection; (B) Representative VEP of DEX-mice injected with vSAM 5 weeks after injection. Light stimulation at 0 ms. (C) Quantitative comparison of negative VEP amplitude in DEX-control (black bar) vs. vSAM-injected DEX (pink bar) mice. N = 4. Av ± SD. * p < 0.05 (Mann Whitney test). (D) The mean difference of VEP amplitude between non-transfected and transfected DEX-mice is shown as the Gardner-Altman estimation plot. The curve indicates the resampled distribution of the mean difference, given the observed data. The mean difference was plotted on floating axes on the right as a bootstrap sampling distribution. The mean difference is depicted as a dot; the 95% confidence interval is indicated by the ends of the vertical error bar. (E) RBPMS immunostained fluorescence images in peripheral and central retina of DEX-treated mice eyes injected with DEX-only and vSAM-treated (following 4 weeks of intracameral injection in post-DEX C57BL/6J mice). (F) Quantification of RGC counts per mm 2 of the retina. RGCs labeled with RGC marker RBPMS, in red, were counted to determine the surviving RGCs. Av ± SD. * p < 0.05 (Mann Whitney test)
SAM-induced lowering of IOP enhances RGC survival under glaucomatous condition
Mice eyes were treated with vehicle or vSAM (following 4 weeks of intracameral injection in post-DEX C57BL/6J mice) in Dexamethasone-treated eyes. Figure 4 E shows the RBPMS immunostained fluorescence images in the peripheral and central retina of DEX-treated mice eyes injected with vehicle or vSAM. RGCs, labeled with RGC marker RBPMS, were counted to determine the RGC survival. Quantification of RGCs in vSAM and vehicle-injected DEX-mice groups was performed by immunostaining the retinal explant using RGC-specific RBPMS antibody. The number of RGC-positive (RBPMS + nuclei) cells was quantified in a retinal flat mount. Quantification of RGC counts per mm 2 of the retina shows higher survival in the vSAM-treated group (Fig. 4 F).
Optical coherence tomography based monitoring of retinal thickness in vSAM-treated mice
Three-dimensional Optical Coherence Tomographic (OCT) imaging was carried out to monitor any changes in retina structure due to elevated IOP (caused by DEX injection) and the effect of intracameral injection of vSAM. ImageJ was used to analyze the SDOCT images. Quantitative comparison of Ganglion cell and Inner plexiform layer (GCIP) thickness measured from B-scan OCT images of retina of wild-type and DEX-induced IOP-elevated mice showed decreased thickness in IOP-elevated mice, which correlated with lower RGC counts (measured by RGC specific RBPMS antibody staining, Fig. 4 F). Further, DEX-induced IOP elevated mice without (-SAM) and with vSAM injection shows no statistically significant difference (Supplementary Fig. 4 ). Furthermore, injection of vSAM in wild-type mice did not cause any detectable change in GCIP thickness, which implies that vSAM injection does not cause any structural damage to the retina in normal or glaucomatous eyes.
Biodistribution of AAV-packaged SAM
The intracamerally injected vSAM mice were euthanized, and the eye and other tissue samples (Mandibular and Mesenteric lymph nodes, testis/ovary, kidney, liver, brain, spleen, lung, and heart) were harvested. The qPCR detection of vector sequences in mice after 5 weeks of intracameral injection of vSAM shows very small or non-detectable quantities of vector in tissues outside of the treated eyes (Supplementary Fig. 5 ).
SAM provides a unique mechanism of action in lowering IOP . SAM, upon mechanical activation, opens a large non-specific pore to release water, ions, and macromolecules [ 19 ]. The SAM molecule is autonomous and does not need partners or energy sources to function. SAM intrinsically senses tension in the membrane and gates in direct response to this mechanical tension. Therefore, the expression of SAM in the TM cell membrane provides a unique mechanism of action for the treatment of glaucoma via the lowering of IOP. Figure S6 describes the unique mechanism of action of SAM-transduced TM, wherein in response to elevated IOP, increased transcellular and paracellular transport decreases IOP. Upon increase in IOP, activation of SAM leads to (i) fluid expulsion through the porous TM cells (transcytosis) into the Schlemm’s canal (SC), as observed by an increased outflow facility in SAM-treated mice; and (ii) cell shrinkage widens intercellular spaces (paracytosis) allowing for increase in aqueous humor outflow facility.
Patient-dependent drug treatment protocols of daily doses of eyedrops or oral tablets are problematic due to poor patient compliance. Researchers are developing non-invasive drug-delivery ocular implants; however, patients will still need constant medication and dosage adjustments due to drug unresponsiveness and disease conditions. In POAG, increased IOP is often associated with increased outflow resistance through the TM. However, the existing surgery/drugs address the improvement of drainage through the conventional outflow pathway [ 22 ]. The most common glaucoma surgery is a trabeculectomy [ 23 ], where an alternative outflow pathway is created by removing a section of the sclera, Schlemm’s canal, and TM and fashioning a filtration bleb out of a conjunctival tissue flap. Other surgical methods include laser peripheral iridotomy [ 24 ], and drainage device implantation [ 25 ]. SAM-based gene therapy exploits the integration of an exogenous macromolecular pressure sensor and outflow actuator into TM cells that target the site of outflow resistance to regulate IOP.
AAV was chosen as the viral serotype for the delivery of SAM to the TM because literature [ 26 ] has shown that AAV2/8 was able to successfully transfect cells of the conventional outflow pathway in mice [ 27 ]. In response to elevated IOP, SAM-transduced TM facilitates increased transcellular and paracellular transport as evidenced in AH outflow facility measurements. This is supported by studies showing that when the contractility of TM cells is inhibited (e.g., by nitric oxide donors and Rho kinase inhibitors [ 28 ]), the cells change shape, thereby creating more intercellular spaces and consequently improving AH outflow through the TM [ 2 ]. At its pressure threshold, SAM can release fluid from TM cells to be cleared through Schlemm’s canal. SAM acts as an ideal drainage valve for TM cells because it is a large non-specific pore that does not need any associated proteins or energy sources to assemble and function. Additionally, the resulting decrease in cell volume and size will widen intercellular spaces and facilitate the paracellular flow of aqueous humor.
SAM-based barogenetic gene therapy for POAG focuses on disease phenotype versus a specific genotype. Most gene therapies aim to compensate for a single aberrant gene by introducing a functional copy of the gene into the cell. The existing studies on identifying the gene(s) linked to Glaucoma and therapeutic interventions are genotype-specific such as the MYOC gene (encoding for myocilin) causing some forms of POAG and Rho-Kinase inhibitor-based therapy [ 6 ]. However, POAG is heterogeneous and poorly genetically characterized; therefore, the introduction of SAM-gene therapy as an exogenous mechanosensitive modulator provides a unique opportunity to bypass specific gene targets. Cellular recovery after SAM activation may trigger several signaling cascades that could improve TM cell function. In bacteria, the expression of heat shock proteins (HSPs) is upregulated after MscL gating during hypo-osmotic shock [ 29 ]. In mouse mutant myocilin-induced glaucoma (a human POAG genotype) the co-expression of protein-folding chaperones (including HSPs) improves outflow facility by preventing mutant myocilin-induced protein aggregation, thereby restoring function to the cells [ 30 ]. Therefore, we hypothesize that if SAM activation triggers HSP expression in mutant myocilin-induced glaucoma TM cells, the AH outflow facility would also be improved.
SAM-based gene therapy has many advantages over drugs and surgical treatments. It would provide an opportunity for a long-term treatment not requiring extensive surgical interventions and repeated dosing for IOP management. The safety and efficacy of AAV-mediated gene therapy have been demonstrated in recent clinical trials and eyes, being self-contained, are ideal targets [ 2 ]. The use of a tropic AAV delivered-SAM and cell-specific promoters can further minimize the risk of undesired side effects. With viral vector-based delivery, the episomal expression of gene encoding SAM-molecule would allow long-term SAM expression. The dysfunctional turnover of proteins in TM cells of POAG will help maintaining the SAM expression over a long period. If turnover issues are encountered over the long-term, the non-viral gene delivery approach [ 31 ] provides a unique opportunity to redose subjects without causing inflammation or immunogenicity. In cases of low SAM expression or in situations that require activation at low ocular pressures, an external ultrasound device [ 32 , 33 ] may be used to stimulate SAM in a non-invasive manner. Though MscL-expressing neurons have been shown to be stimulated by ultrasound [ 34 ], it is yet to be shown if heterologously-expressed MscL in TM cells can be modulated by external ultrasound to further enhance the outflow facility.
Several AAV gene therapy preclinical studies have focused on disease phenotype to treat glaucoma [ 35 ]. Table S1 lists the Gene target for IOP lowering, Mechanism of action, Genetic manipulation, Gene delivery method, Gene type, and Animal model of Glaucoma. One of the targets involves enzymes that are directly or indirectly involved in enhancing/degrading extracellular matrix by Matrix metalloproteinases (MMPs). Intracameral injection of scAAV2-MMP1 driven by a GRE promoter has been shown to reduce IOP in steroid-induced sheep model [ 36 ]. Increased expression of proteins that can induce MMP expression also has been shown to significantly reduce IOP. Examples include plasminogen activator tissue (PLAT) [ 37 ], Cycloexogenase-2 [ 38 ], prostaglandin F synthase (PGFS) [ 39 ], COX-2 and prostaglandin F receptor ( PTGFR ) [ 40 ]. Another mechanism of IOP reduction by gene therapy involves disruption of the actin network. Downregulation of RhoA kinase by either siRNA or scAAV2 expressing the dominant-negative RhoA has been shown to cause significant reduction in IOP [ 36 , 41 ]. In contrast, inhibiting RhoA kinase signaling by expression of Clostridium botulinum C3 exoenzyme mediating the ADP-ribsoylation of GTP-binding proteins RhoA, B and C leading to the inhibition of the Rho signaling pathway, was efficient in enhancing AH outflow [ 42 ]. In addition, upregulation of caldesmon protein that is known to disrupt actin network also enhanced AH outflow [ 43 ]. IOP has also been shown to be lowered by modulating AH formation via downregulation of aquaporin-1 [ 44 ], carbonic anhydrases [ 45 ], β2-adrenergic receptor [ 46 , 47 ], P2Y2 receptor [ 48 ], and endothelial nitric oxide synthase [ 49 ]. Further, increased expression of stanniocalcin-1, a stress-response protein using scAAV2 has been shown to lower IOP for 6 months in mice [ 50 ]. In the current study, we utilized mechanosensitive bacterial channels’ transduction in cells of trabecular meshwork to act as a tension activated pressure-release-valve.
Although the mechanosensitive channels derived from different bacteria are highly homologous, they exhibit distinct differences in their structure and function that were capitalized upon to create a SAM variant that would be useful in the treatment of open-angle glaucoma. SAM and its variants are activated at physiologically relevant pressures around normal human IOP of 10–21 mmHg. As SAM activity is triggered solely by the transduction of mechanical force through lipid-protein interactions, amino acid residues that form those interfaces are critical [ 12 ]. The barogenetic approach presented in the manuscript can be further optimized by phylogenetic selection of pressure-sensing and modulating channels that exists in nature along with mutation(s) in the molecular system. The screening of channels can be conducted using pressure clamp electrophysiology by application of appropriate pressure modulation as occurs in the eye during elevated intraocular pressure due to glaucoma, pulsatile ocular blood flow or rubbing of eyes. While the data presented here shows IOP lowering over 8 weeks, the efficacy of SAM therapy in modulating IOP over longer term needs to be evaluated. Further, the translational impact of SAM therapy will depend on transduction efficacy of intracamerally delivered vSAM in human. This will require optimization of the delivery vector (rAAV vs. scAVV) and determination of therapeutic dose for optimal efficacy outcome that does not compromise safety. The eye being immune-privileged allow tolerance to viral vector-based gene therapy with a lower risk of immune-rejection compared to other organs. In case of safety events or requirement of redosing due to loss of gene expression because of turnover in TM, non-viral gene delivery [ 51 , 52 ] may be utilized. Successful translation of SAM barogenetic gene therapy from research to clinical practice will have potential benefits to patients with abnormal pressure dynamics.
Conclusions
The SAM technology described in this manuscript exploits the integration of an exogenous macromolecular pressure sensor and outflow actuator into TM cells to regulate IOP. The development of a safe and effective long-lasting gene therapy for glaucoma has potential to improve the lives of many individuals. While the technology described in this manuscript shows for the first-in-class control of elevated eye pressure in-vivo by use of a bacterial mechanosensitive channel, the SAM system has to be further characterized and optimized for its use in clinical settings. The pressure sensing and modulation approach may find applications within other organs and therapeutic areas involving abnormal changes in osmotic or mechanical pressure.
Materials and methods
Ethics statement.
The experimental protocols were approved by the Institutional Animal Care and Use Committee and all experiments were performed in accordance with relevant guidelines and regulations.
Cloning of SAM
The EcMscL gene from the BL21 (DE3) E.coli strain, which encodes for a 136 amino acid monomer, was retrieved and codon optimized for mammalian cell expression. SAM gene sequence is a double mutant (I113L, I70E) of EcMscL. The gene sequence was codon optimized for mammalian expression before synthesis. The gene was put under the control of a TM-specific promoter. Several studies have shown that in the eye matrix Gla protein (MGP) is preferentially expressed in TM cells and its promoter sequence has been used to target gene expression. The MGP promoter sequence (pMGP) located on chromosome 12 was identified by using the primers [ 18 ] to run an alignment search against the reverse complement strand of the human genome GRCh38.p12. The resulting 576 bp promoter sequence was placed on the 5’ end of the SAM gene. A fused fluorescent reporter was chosen to act as a visual marker of gene expression and membrane localization. As SAM is a homo-oligomer and needs to assemble into a functional complex postranslationally, a strictly monomeric fluorescent protein had to be chosen to avoid any disruptive interactions. To this end, the monomeric fluorescent protein, mCherry, was C-terminally fused to SAM. The gene was inserted into a pAAV-MCS vector backbone by restriction digestion and ligation (T4 DNA Ligase).
Production of AAV carried SAM
AAV carried SAM production was carried out using the triple plasmid transfection method. HEK293 cells were cultured and transfected with a mixture of DNA (RepCap, transfer, and helper plasmids), polyethylenimine, polyethylene glycol (PEG), and Dulbecco’s Modified Eagle Medium (DMEM). After incubation at 37 °C for 72 h, cells were trypsinized and lysed. The virus was purified using FPLC chromatography [ 53 ]. Viral vectors were resuspended in PBS and titrated to a concentration of 1 × 10 13 vg/ml. Vector genome copies were measured by qPCR amplification and SDS-PAGE gel electrophoresis was used to determine sample purity.
HEK293 cell culture
HEK293 cells were grown in flasks in 1.5 mL of Dulbecco’s modified Eagle’s medium (DMEM) with 4.5 g/L glucose, L-glutamine and sodium pyruvate, 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin sulfate and incubated in a humidified 5% CO 2 environment at 37 °C. A day before transfection, cells were plated in 35 mm Petri dishes in 1.5 ml of media and incubated at 37 °C until ~ 80% confluency.
Corneoscleral culture from rat
Following established dissection procedure [ 54 ], the anterior segment from the embryonic eyes of the rat was cut into small explants. After cellular dissociation1.33% collagenase (30–45 min), followed by Trypsin-EDTA (20 min), the corneoscleral cells were seeded onto a poly-D-lysine pre-coated Petri dish. 10% fetal bovine serum was used for the TM cell culture and was changed every 2–3 days. The TM cells were cultured at 37 °C in 5% CO 2 in a humidified atmosphere.
Human TM cell culture
Primary human TM cells (Cell Applications Inc), maintained in DMEM supplemented with 10% FBS and antibiotics, were cultured in Petri dishes. Passages 1 through 4 were used for quantification of transduction efficiency. Analysis of myocilin expression in these cells following dexamethasone treatment showed a reliable increase as reported in the literature [ 54 ].
In-vitro transfection
jetPRIME (Polyplus Transfection) was used to deliver 2 µg of SAM-mCherry plasmid DNA to each dish. The plasmid DNA was added to 200 µl of jetPRIME buffer and then vortexed for 10 s. Then, 8 µl of jetPRIME was added and the solution was left to sit at room temperature for 10 min. The solution was then added to the cells and the sample was incubated at 37 °C for 2 h. After 2 h, the growth medium was exchanged for fresh media and incubated at 37 °C for another 48 h. At this time, fluorescence microscopy of live cells was used to confirm the successful transfection and expression of the fluorescent reporter (mCherry).
Fluorescence Confocal Microscopy
Confocal Fluorescence imaging (Olympus FluoView 1000) on an upright microscope platform was carried out to confirm the successful transfection and expression of the fluorescent reporter (mCherry) linked to the SAM gene in cells.
Patch clamp Electrophysiology
The functioning and response of SAM-expressing cells to osmotic pressure were measured by recording electrophysiological measurements from whole cell patches of HEK293 and TM cells. The patch-clamp recording setup includes an inverted Nikon fluorescence microscope (TS 100) platform using an amplifier system (Axon Multiclamp 700B, Molecular Devices). Micropipettes were pulled using a two-stage pipette puller (Narshinghe) to attain resistance of 3 to 5 MΩ when filled with a solution containing (in mM) 130 K-Gluoconate, 7 KCl, 2 NaCl, 1 MgCl 2 , 0.4 EGTA, 10 HEPES, 2 ATP-Mg, 0.3 GTP-Tris and 20 sucrose. The micropipette-electrode was mounted on a micromanipulator. The extracellular solution contained (in mM): 150 NaCl, 10 Glucose, 5 KCl, 2 CaCl 2 , and 1 MgCl 2 was buffered with 10 mM HEPES (pH 7.3). The transfected cells were identified by observing the mCherry expression under green excitation light. Inward currents were measured while holding cells in a voltage clamp at -70 mV. The electrophysiological signals from the amplifier were digitized using Digidata 1440 (Molecular devices), interfaced with patch-clamp software (Clampex, Molecular Devices). pClamp 10 software was used for data analysis. SAM channel activity was induced by subjecting the patched cell to a hypo-osmotic shock by adding of 20% v/v water. A stable gigaohm seal was achieved and the current traces show channel activity only after the hypo-osmotic shock.
Automated pressure-clamp Electrophysiology
The functioning and response of SAM-expressing cells to pressure clamp were assessed by recording electrophysiological signals from whole-cell patches using the Port-a-Patch system (Nanion, Germany). Planar patch-clamp chips with a chip resistance of 2–3.5 MΩ were used. The internal solution consisted of 10 mM KCl, 10 mM NaCl, 110 mM K-Fluoride, 10 mM EGTA, 10 mM HEPES/KOH, and pH 7.2. The external solution consisted of 4 mM KCl, 140 mM NaCl, 1 mM MgCl 2 , 2 mM CaCl 2 , 5 mM D-Glucose monohydrate, 10 mM HEPES/NaOH, Ph 7.4. The seal resistance was typically 1GΩ. The pressure was introduced by SuctionControl Pro (Nanion). The electrophysiological signals from the amplifier (Axon Multiclamp 700B, Molecular Devices) were digitized using Digidata 1440 (Molecular Devices).
Measurement of visually evoked potential (VEP)
The mice were dark-adapted overnight and anesthetized with an intraperitoneal injection of a mixture of Ketamine (90 mg/kg), Xylazine (10 mg/kg), and Acepromazine (0.5 mg/kg). The VEP measurement was carried out in the dark using dim red light. Using the surgical tools, the skull was exposed, and craniotomy was performed by drilling a hole for the recording electrode to access the V1 (AP = − 3.0 mm, and ML = + 2.5 mm relative to bregma) area of the Visual cortex. The mouse was placed on the stereotaxic unit and the ground electrode was subcutaneously placed in the tail. The reference electrode was placed at AP = + 2.5 mm and ML = − 1.5 mm. Light flash-VEPs were elicited by a white LED Stimulator providing stimulus with a stimulus rate of 1 Hz and stimulus duration of 1 ms. Signals were amplified through a Built-In Bias Drive Amplifier, Analog-to-digital converters (ADCs) with a built-in programmable gain amplifier (PGA). A high-pass filter at 5 Hz and a low-pass filter at 250 Hz with a 60 Hz notch filter. Acquisitions were performed at a 1 kHz sampling rate. VEPs from a sequence of 15 strobe light flashes were averaged to obtain the final waveform.
Spectral-domain (SD) optical coherence tomography imaging
Optical Coherence Tomographic (OCT) imaging is a standard ophthalmic assessment tool that provides quantitative measurements of the anterior segment and retina instead of subjective evaluation. Optical sectioning/imaging using SDOCT was carried out to monitor any changes in ocular structure due to intracameral injection of vSAM or vehicle control. Animals were anesthetized using a mixture of Ketamine/Xylazine/Acepromazine. For dilating the pupil, a drop of Tropicamide was topically applied to the eye. SDOCT images of the retina after intracameral injection of vSAM in mice were compared to the images before injection. The retinal thickness measurements were made from the OCT B-scan images using ImageJ.
Experimental animal groups
Dexamethasone-treated adult (> 8 weeks old) C57BL/6J mice (strain 000664, The Jackson Laboratory) were used as a glaucoma model [ 20 ]. Wild-type mice were used for monitoring the effect of SAM injection in DEX-induced IOP-elevated mice. Elevated IOP in mice was achieved by topical application of 0.1% dexamethasone (DEX), 3 times daily. For monitoring the treatment effect of vSAM, 2 different wild-type mice groups were used. Group 1 consisted of DEX-treated control mice (without SAM injection). Group 2 consisted of mice with vSAM injection at day 1 in DEX mice, and Group 3 comprised of vSAM injection at 4 weeks after initiation of DEX application. DEX treatment protocol (0.1% dexamethasone topical, 3 times daily, 7 days a week) was followed for all three Arms during the whole course of the study.
Intracameral injections
Anesthetized mice (intraperitoneal injection with a mixture of 90 mg/kg ketamine, 10 mg/kg xylazine, and 0.5 mg/kg acepromazine) were injected with virus intracamerally. Under a surgical microscope, a 34 gauge needle was inserted into the cornea, anterior of the iridocorneal angle, and vSAM was delivered at an infusion rate of 1 µl/min. After the procedure, antibiotics were applied to the mouse eye.
IOP monitoring
For IOP measurement, the mouse was placed on a wire cage and allowed ~ 1 min of resting time. The tonometer (Tonovet Plus, iCARE) was loaded with a new probe allowing it for self-calibration. The Tonovet was positioned parallel to the eye with a distance of about half an inch and aimed at the center of the cornea. The IOP was measured in the afternoons. Three trials of IOP measurement were carried out, each trial consisting of 6 readings.
Measurement of aqueous outflow facility
The apparatus for assessments of aqueous humor hydrodynamics is shown in Suppl. Figure 3 A. To measure the aqueous outflow facility, the mice were anesthetized. The anterior chamber of the mouse eye is cannulated by using a 32-gauge steel needle (Suppl. Figure 3 B), which was connected to a flow-through pressure transducer for the continuous determination of pressure within the system. The pressure–time trace was obtained from a single experiment. In the anesthetized animal, the pressure was monitored continuously as the Flow rate was set at different rates (Suppl. Figure 3 C). The pump was stopped, and the circuit opened to the manometer, which was then rapidly lowered to re-establish baseline pressure. After this, the circuit was closed again, the animal was euthanatized, perfusion was resumed, and the pressure was monitored to determine the outflow facility.
Harvesting tissue
Mice were euthanized 35 d post-injection in a CO 2 gas chamber and spinal dislocation. The eyes were fixed in 4%PFA at 4 °C overnight, rinsed with PBS, and kept frozen. For biodistribution studies, other vital tissues were kept frozen in the 1.8 ml cryovials and stored at -80 °C. Each vial was properly labeled with an animal identification number, date of extraction, and name of the organ.
Tissue sectioning
Enucleated mouse eyeballs were fixed in 4% PFA at 4 °C overnight followed by storage in 1X PBS. The eyeballs were treated with 30% sucrose and embedded in an optimal cutting temperature (OCT) embedding compound (Tissue-Tek OCT; Electron Microscopy Sciences, Hatfield, PA). For immunostaining, the eyeballs were cryo-sectioned at 20 μm thickness (Microtome, Microm).
Western blot analysis
Antibodies against fibronectin and mCherry were used to test for protein localization. After fluorescence imaging, cells were lysed, the membrane and cytoplasmic fractions separated by centrifugation, and run on an SDS-PAGE gel. Protein bands were transferred to a nitrocellulose/PVD membrane for immunostaining.
Immunostaining and imaging
For immunostaining, the cells, flat mount, and tissue slices were fixed in 4% PFA at 4 o C, which was replaced with 1X PBS. The nonspecific binding of antibodies was blocked by 4% serum for 60 minutes and washed with washing solution three times. The samples were then incubated at 4°C overnight with anti-mCherry (1: 500 dilution), anti-Myocilin, anti-Collagen-IV, anti-PAI-1, anti-Fibronectin (TM-markers), or RBPMS (RGC marker). After washing the samples with 0.5% TritonX-100 solution three times, secondary antibodies were added to the samples for incubation (one hour at room temperature). The samples were stained with nuclear stain DAPI (4’, 6-diamidino-2-phenylindole) dye at room temperature for 30 min, rinsed in PBS, and then mounted onto slides in a mounting medium.
Images were taken by confocal microscope (Olympus Fluoview FV1000, Olympus, Center Valley, PA). Image analysis was performed using ImageJ software. Expression of SAM-reporter (mCherry) in TM was carried out in animals injected with vSAM and was compared to control.
DNA extraction and qPCR
Genomic DNA was extracted from tissue samples using the Phenol/chloroform DNA extraction technique [ 17 ] and using the Thermofisher Scientific GeneJET Genomic DNA Purification Kit (cat# K0722) according to the manufacturer’s protocol. qPCR was performed using Takara AAVpro™ Titration kit standard (cat# 6233) and Fisher Scientific Company’s Master Mix, qPCR (Applied Biosystems), and Power Up SYBR Green Master Mix (cat# A25776). 50X Primer Mix was prepared as follows: AAV Forward ITR Primer 5 µl, AAV Reverse ITR primer 5 µl, water 15 µl. The qPCR reaction mix consisted of: SYBR green Premix 12.5 µl, 50X Primer Mix 0.5 µl, water 7 µl, and template DNA (~ 10 ng) 5 µl. Real-time PCR was performed on the Applied Biosystems QuantStudio 3 real-time PCR System (Applied Biosystems) using assays specific for ITR. Samples were analyzed in duplicate for vector copy number/ng DNA by the Absolute Quantification method using standard curves. Preparation of the standard curve was performed following the manufacturer reference guide.
Image processing and analysis were performed using NIH ImageJ software. GraphPad Prism was used to analyze the data. The data were plotted as mean ± SD or SEM. Statistically significant difference analyses were carried out by t-test. In case of low sample size ( N < 6), non-parametric Mann Whitney test was performed. P < 0.05 was considered statistically significant.
Data availability
All data are available in the main text or the supplementary materials.
Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90.
Article PubMed Google Scholar
Kaufman PL, Mohr ME, Riccomini SP, Rasmussen CA. Glaucoma drugs in the pipeline. Asia-Pacific J Ophthalmol. 2018;7:345–51.
CAS Google Scholar
Wang C, Li L, Liu Z. Experimental research on the relationship between the stiffness and the expressions of fibronectin proteins and adaptor proteins of rat trabecular meshwork cells. BMC Ophthalmol. 2017;17:268.
Article PubMed PubMed Central Google Scholar
Raghunathan VK, et al. Glaucomatous cell derived matrices differentially modulate non-glaucomatous trabecular meshwork cellular behavior. Acta Biomater. 2018;71:444–59.
Article CAS PubMed PubMed Central Google Scholar
Yoo H et al. Simvastatin attenuates glucocorticoid-induced human trabecular meshwork cell dysfunction via YAP/TAZ inactivation. Curr Eye Res, 1–14 (2023).
Acott TS, Vranka JA, Keller KE, Raghunathan V, Kelley MJ. Normal and glaucomatous outflow regulation. Prog Retin Eye Res. 2021;82:100897.
Bermudez JY, Montecchi-Palmer M, Mao W, Clark AF. Cross-linked actin networks (CLANs) in glaucoma. Exp Eye Res. 2017;159:16–22.
Challa P, Arnold JJ. Rho-kinase inhibitors offer a new approach in the treatment of glaucoma. Expert Opin Investig Drugs. 2014;23:81–95.
Article CAS PubMed Google Scholar
Hayreh SS, Podhajsky P, Zimmerman MB. Beta-blocker eyedrops and nocturnal arterial hypotension. Am J Ophthalmol. 1999;128:301–9.
Hoy SM. Netarsudil ophthalmic solution 0.02%: first global approval. Drugs. 2018;78:389–96.
Youngblood H, Hauser MA, Liu Y. Update on the genetics of primary open-angle glaucoma. Exp Eye Res. 2019;188:107795.
Zukerman R, Harris A, Verticchio Vercellin A, Siesky B, Pasquale LR, Ciulla TA. Molecular genetics of glaucoma: subtype and ethnicity considerations. Genes. 2020;12:55.
Jain A, et al. CRISPR-Cas9–based treatment of myocilin-associated glaucoma. Proc Natl Acad Sci. 2017;114:11199–204.
Ryskamp DA, et al. TRPV4 regulates calcium homeostasis, cytoskeletal remodeling, conventional outflow and intraocular pressure in the mammalian eye. Sci Rep. 2016;6:1–15.
Article Google Scholar
Sukharev SI, Blount P, Martinac B, Blattner FR, Kung C. A large-conductance mechanosensitive channel in E. Coli encoded by mscL alone. Nature. 1994;368:265–8.
Blount P, Iscla I. Life with bacterial mechanosensitive channels, from discovery to physiology to pharmacological target. Microbiol Mol Biol Rev. 2020;84:e00055–00019.
Heureaux J, Chen D, Murray VL, Deng CX, Liu AP. Activation of a bacterial mechanosensitive channel in mammalian cells by cytoskeletal stress. Cell Mol Bioeng. 2014;7:307–19.
Liton PB, Liu X, Stamer WD, Challa P, Epstein DL, Gonzalez P. Specific targeting of gene expression to a subset of human trabecular meshwork cells using the chitinase 3-like 1 promoter. Investig Ophthalmol Vis Sci. 2005;46:183–90.
Perozo E, Cortes DM, Sompornpisut P, Kloda A, Martinac B. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature. 2002;418:942–8.
Zode GS, et al. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J Clin Investig. 2014;124:1956–65.
Shepard AR, Millar JC, Pang I-H, Jacobson N, Wang W-H, Clark AF. Adenoviral gene transfer of active human transforming growth factor-β2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Investig Ophthalmol Vis Sci. 2010;51:2067–76.
Sharif NA. Therapeutic drugs and devices for tackling ocular hypertension and glaucoma, and need for neuroprotection and cytoprotective therapies. Front Pharmacol. 2021;12:729249.
Watson PG, Grierson I. The place of trabeculectomy in the treatment of glaucoma. Ophthalmology. 1981;88:175–96.
Ang LP, Aung T, Chew PT. Acute primary angle closure in an Asian population: long-term outcome of the fellow eye after prophylactic laser peripheral iridotomy. Ophthalmology. 2000;107:2092–6.
Greenfield DS, Tello C, Budenz DL, Liebmann JM, Ritch R. Aqueous misdirection after glaucoma drainage device implantation. Ophthalmology. 1999;106:1035–40.
Mao W, Liu Y, Mody A, Montecchi-Palmer M, Wordinger RJ, Clark. A. F. characterization of a spontaneously immortalized bovine trabecular meshwork cell line. Exp Eye Res. 2012;105:53–9.
Johnson TV, Tomarev SI. Rodent models of glaucoma. Brain Res Bull. 2010;81:349–58.
Wang SK, Chang RT. An emerging treatment option for glaucoma: rho kinase inhibitors. Clin Ophthalmol (Auckland NZ). 2014;8:883.
Jones SE, Naik RR, Stone MO. Use of small fluorescent molecules to monitor channel activity. Biochem Biophys Res Commun. 2000;279:208–12.
Kasetti RB, Phan TN, Millar JC, Zode GS. Expression of mutant myocilin induces abnormal intracellular accumulation of selected extracellular matrix proteins in the trabecular meshwork. Investig Ophthalmol Vis Sci. 2016;57:6058–69.
Article CAS Google Scholar
Hangai M, Tanihara H, Honda Y, Kaneda Y. Introduction of DNA into the rat and primate trabecular meshwork by fusogenic liposomes. Investig Ophthalmol Vis Sci. 1998;39:509–16.
Coleman DJ, et al. Therapeutic ultrasound in the treatment of glaucoma: II. Clinical applications. Ophthalmology. 1985;92:347–53.
Silverman RH, Vogelsang B, Rondeau MJ, Coleman DJ. Therapeutic ultrasound for the treatment of glaucoma. Am J Ophthalmol. 1991;111:327–37.
Ye J, et al. Ultrasonic control of neural activity through activation of the mechanosensitive channel MscL. Nano Lett. 2018;18:4148–55.
Castro B, Steel JC, Layton CJ. AAV-mediated gene therapies for glaucoma and uveitis: are we there yet? Expert Rev Mol Med. 2024;26:e9.
Borrás T, Buie L, Spiga MG. Inducible scAAV2. GRE. MMP1 lowers IOP long-term in a large animal model for steroid-induced glaucoma gene therapy. Gene Ther. 2016;23:438–49.
Kumar S, Shah S, Tang HM, Smith M, Borrás T, Danias J. Tissue plasminogen activator in trabecular meshwork attenuates steroid induced outflow resistance in mice. PLoS ONE. 2013;8:e72447.
Barraza RA, McLaren JW, Poeschla EM. Prostaglandin pathway gene therapy for sustained reduction of intraocular pressure. Mol Ther. 2010;18:491–501.
Lee ES, et al. Prospects for lentiviral vector mediated prostaglandin F synthase gene delivery in monkey eyes in vivo. Curr Eye Res. 2014;39:859–70.
Chern KJ, Nettesheim ER, Reid CA, Li NW, Marcoe GJ, Lipinski DM. Prostaglandin-based rAAV-mediated glaucoma gene therapy in Brown Norway rats. Commun Biology. 2022;5:1169.
Liu Q, Wu K, Qiu X, Yang Y, Lin X, Yu M. siRNA silencing of gene expression in trabecular meshwork: RhoA siRNA reduces IOP in mice. Curr Mol Med. 2012;12:1015–27.
Liu X, et al. The effect of C3 transgene expression on actin and cellular adhesions in cultured human trabecular meshwork cells and on outflow facility in organ cultured monkey eyes. Mol Vis. 2005;11:1112–21.
PubMed Google Scholar
Gabelt BAT, Kaufman PL. Bimatoprost for glaucoma therapy: pharmacology, clinical efficacy and controversy. Expert Rev Ophthalmol. 2006;1:141–58.
Wu J, et al. Gene therapy for glaucoma by ciliary body aquaporin 1 disruption using CRISPR-Cas9. Mol Ther. 2020;28:820–9.
Jimenez A, Sesto A, Pintor J, Mediero A, Peral A, de Buitrago GG. RNAi: a new strategy for treating ocular hypertension silencing carbonic anhydrases. Investig Ophthalmol Vis Sci. 2006;47:405–405.
Google Scholar
Loma P, Guzman-Aranguez A, de Lara MJP, Pintor J. Beta2 adrenergic receptor silencing change intraocular pressure in New Zealand rabbits. J Optometry. 2018;11:69–74.
Moreno-Montañés J, et al. Phase I clinical trial of SYL040012, a small interfering RNA targeting β-adrenergic receptor 2, for lowering intraocular pressure. Mol Ther. 2014;22:226–32.
Martin-Gil A, de Lara MJP, Crooke A, Santano C, Peral A, Pintor J. Silencing of P2Y2 receptors reduces intraocular pressure in New Zealand rabbits. Br J Pharmacol. 2012;165:1163–72.
You Y, et al. Progesterone promotes endothelial nitric oxide synthase expression through enhancing nuclear progesterone receptor-SP-1 formation. Am J Physiol Heart Circ Physiol. 2020;319:H341–8.
Roddy GW, et al. Transgene expression of Stanniocalcin-1 provides sustained intraocular pressure reduction by increasing outflow facility. PLoS ONE. 2022;17:e0269261.
Batabyal S, Gajjeraman S, Tchedre K, Dibas A, Wright W, Mohanty S. Near-Infrared laser-based spatially targeted nano-enhanced optical delivery of therapeutic genes to degenerated retina. Mol Therapy Methods Clin Dev. 2020;17:758–70.
Batabyal S, Kim S, Wright W, Mohanty S. Layer-specific nanophotonic delivery of therapeutic opsin-encoding genes into retina. Exp Eye Res. 2021;205:108444.
Burova E, Ioffe E. Chromatographic purification of recombinant adenoviral and adeno-associated viral vectors: methods and implications. Gene Ther. 2005;12(Suppl 1):S5–17.
Keller KE, et al. Consensus recommendations for trabecular meshwork cell isolation, characterization and culture. Exp Eye Res. 2018;171:164–73.
Download references
Acknowledgements
The authors would like to thank Dr. Cameron Millar, UNT Health Science Center for help with the outflow measurement setup.
National Eye Institute R44EY033626.
Author information
Authors and affiliations.
Nanoscope Technologies, LLC, 1312 Brown Trail, Bedford, TX, 76022, USA
Samarendra Mohanty, Subrata Batabyal, Chinenye Idigo, Darryl Narcisse, Sanghoon Kim, Houssam Al-Saad, Michael Carlson, Kissaou Tchedre & Adnan Dibas
You can also search for this author in PubMed Google Scholar
Contributions
SM designed and supervised the experiments. SB performed patch clamp experiments, imaging, and data analysis. CI designed the construct, DN participated in plasmid purification/production of the vector, SK performed the OCT imaging, HA performed IOP measurement, MC performed electrophysiology (VEP) assessments, and KT performed sectioning, and biodistribution. AD performed cell culture and Western blot. All authors participated in discussion, and data analysis and contributed to the preparation of the manuscript.
Corresponding author
Correspondence to Samarendra Mohanty .
Ethics declarations
Competing interests.
SM has an equity interest in Nanoscope Technologies, LLC.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Mohanty, S., Batabyal, S., Idigo, C. et al. Engineered sensor actuator modulator as aqueous humor outflow actuator for gene therapy of primary open-angle glaucoma. J Transl Med 22 , 791 (2024). https://doi.org/10.1186/s12967-024-05581-1
Download citation
Received : 22 May 2024
Accepted : 06 August 2024
Published : 28 August 2024
DOI : https://doi.org/10.1186/s12967-024-05581-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pressure regulator
- Intraocular pressure
- Gene therapy
- Barogenetics
Journal of Translational Medicine
ISSN: 1479-5876
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

The Cure is in Sight.
Glaucoma research.

Our multidisciplinary consortium of researchers is working together to speed the cure for glaucoma and restore vision.

Catalyst for a Cure principal investigators report on their latest challenges and progress.

Glaucoma Research Foundation provides seed money for creative pilot research projects that hold promise.

Glaucoma Events

The Annual Gala is part of Glaucoma 360, a series of events uniting research, industry, and philanthropy with one mission — to find a cure for glaucoma.

The Patient Summit conference helps patients understand and live with glaucoma by highlighting advances in treatment options and and reviewing practical information.

New Horizons Forum unites key clinical, industry, financial, and FDA leaders in a unique exchange on research innovation and advances in glaucoma treatment.

For Eyecare Professionals

To help you manage the vast amount of glaucoma information online, we provide an organized list of helpful links. You’ll find guidance for support groups , free booklets , finding a doctor , and more.

COMMENTS
The U.S. Food and Drug Administration has approved a new treatment for glaucoma, iDose® TR (travoprost intracameral implant, 75 mcg). iDose TR is a first-of-its kind treatment that is designed to provide up to three years of 24/7, continuous drug therapy directly inside the eye, helping people with glaucoma take control over the elevated eye pressure associated with this vision disease.
The most extensive genome-wide association study of glaucoma to date compared the genes of 34,179 people with the disease to 349,321 control participants. It enabled an international consortium of researchers to identify 44 new gene loci ("genetic street addresses" that denote a specific location on a gene). It confirmed 83 previously ...
Three new treatments for dry eye disease and a new procedure and remote monitoring platform for glaucoma are due to debut in 2024, with more in the pipeline. ... Image 2: Glaucoma Research Foundation.
Catalyst for a Cure: 2024 Research Update. The Catalyst for a Cure research teams are generating insights and results as they seek new treatments and cures for glaucoma and other neurodegenerative diseases. Glaucoma Research Foundation currently has two groundbreaking Catalyst for a Cure initiatives underway at once. The two research teams are ...
Xen-DS: a novel technique of ab externo Xen implantation augmented with a modified deep sclerectomy for surgical treatment of glaucoma. A. Elbably. J. Richardson-May. A. Jacob. Research 21 May ...
The treatment had multiple beneficial effects on the eye. First, it promoted nerve regeneration following optic-nerve injury in mice with damaged optic nerves. Second, it reversed vision loss in animals with a condition mimicking human glaucoma. And third, it reversed vision loss in aging animals without glaucoma.
Glaucoma Research Foundation is dedicated to scientific research that deepens our understanding of glaucoma, generates new treatments, and will ultimately lead to a cure. Catalyst for a Cure (CFC) is Glaucoma Research Foundation's flagship research program. We are currently funding two CFC research teams.
June 29, 2023. Reviewed by Preeti Subramanian, PhD. In light of positive results from a BrightFocus-funded Phase I study of an innovative glaucoma therapy involving eye implants, a Phase 2 clinical trial of this exciting potential treatment is currently enrolling patients. The study is testing an implant that delivers a steady stream of growth ...
News Release. Thursday, July 22, 2021. Scientists discover gene therapy provides neuroprotection to prevent glaucoma vision loss. An NIH-funded research project found that calcium modulator CaMKII protects the optic nerve in mice, opening the door to new sight-saving therapy.
Scientifically reviewed by: Preeti Subramanian, PhD A team of National Glaucoma Research (NGR) scientists has linked a genetic mutation with vision damage in glaucoma that points to the possibility of an entirely new treatment method targeting specialized glial cells. The discovery, published in Stem Cell Reports, could work in addition to the standard glaucoma treatment approach of using eye ...
RGC replacement therapy shows potential as a new therapeutic approach for treating glaucoma. Although there are challenges to overcome regarding scaling up RGC production and achieving reliable ...
Grantee. Researchers at Indiana University School of Medicine are using a novel approach to hopefully develop a new therapy for glaucoma, a complex disease that eventually leads to blindness, thanks to a new five-year, $2 million R01 grant from the National Eye Institute. The project, led by Tasneem Sharma, Ph.D. is called "Therapeutic ...
Glaucoma is a progressive disease characterized by damage to the optic nerve and visual loss which, if left untreated, can lead to blindness [ 1, 2, 3 ]. Glaucoma may affect activities of daily ...
The future of glaucoma care. Research in glaucoma continues to improve our understanding of the causes of disease and develop more targeted and personalized treatments. ... loss of nerve cells (of the retina and optic nerve), called neuroprotection, show promise. Researchers are studying new drugs, drug delivery systems, and innovations to make ...
Glaucoma Research Foundation is dedicated to scientific research that deepens our understanding of glaucoma, generates new treatments, and will ultimately lead to a cure. ... Glaucoma Research Foundation's latest research initiative targets the shared roots of conditions that occur when neurons deteriorate and die.
Using gene editing, the scientists in the study developed new models of glaucoma in mice that resembled primary congenital glaucoma. By injecting a new, long-lasting and non-toxic protein treatment (Hepta-ANGPT1) into mice, the scientists were able to replace the function of genes that, when mutated, cause glaucoma. ... and Research to Prevent ...
Glaucoma can be defined as a progressive optic neuropathy that induces optic disc cupping and retinal ganglion cell apoptosis. 1 As the world's leading cause of irreversible blindness, the disease currently affects 3.5% of individuals aged between 40 and 80 years. The incidence of glaucoma is increasing, together with life expectancies, in resource-limited countries, and nearly 112 million ...
Regenerative therapies for the eyes could help to save vision in people with glaucoma, macular degeneration and damaged corneas. ... Westlake University is a new type of non-profit research ...
FULL STORY. A new, detailed genetic roadmap of glaucoma -- the world's leading cause of irreversible blindness -- will help researchers develop new drugs to combat the disease, by identifying ...
Indiana University School of Medicine researchers have identified a new therapeutic target that could lead to more effective treatment of glaucoma. Glaucoma is a neurodegenerative disease that ...
Glaucoma Research Foundation is dedicated to scientific research that deepens our understanding of glaucoma, generates new treatments, and will ultimately cure a disease that is the second leading cause of blindness. Research takes resources. That's why, for more than 40 years, we have rallied financial support for science with the potential ...
Importance: Selective laser trabeculoplasty (SLT) is becoming the recommended first choice in the treatment of open-angle glaucoma (OAG). However, whether repeat SLT can be recommended regardless of initial response remains controversial. Objective: To assess the potential of OAG and ocular hypertension (OHT) undergoing repeat laser to respond favorably to SLT, termed responsiveness to SLT.
Glaucoma 360 is a series of three days of annual events — hosted by Glaucoma Research Foundation — aiming to prevent vision loss from glaucoma and speed the cure. New Research from GRF-funded Investigators Reports Discovery of New Type of Neuron in the Eye
A new Spanish study found that exercise, especially aerobic exercise, may help modulate intraocular pressure (IOP) and may serve as a complementary therapy for patients with glaucoma, according to the lead author Daniel González-Devesa, PhD, from the Faculty of Physical Activity and Sports Sciences, Universidad de León, León, Spain.. Their rationale for delving into this topic is the recent ...
Glaucoma, a blinding eye disease with optic neuropathy, is usually associated with elevated intraocular pressure (IOP). The currently available pharmacological and surgical treatments for glaucoma have significant limitations and side effects, which include systemic reactions to medications, patient non-compliance, eye infections, surgical device failure, and damage to the eye. Here, we ...
To help you manage the vast amount of glaucoma information online, we provide an organized list of helpful links. You'll find guidance for support groups, free booklets, finding a doctor, and more. Our mission: Cure glaucoma and restore vision through innovative research. Whether you need a doctor, support group, or a brochure to share, we ...