- Introduction
- Conclusions
- Article Information
eTable 1. Health Deficits of the Frailty Index in the UK Biobank Cohort
eTable 2. Association of Wine Preference and Drinking During Meals With Mortality in Older Drinkers From the UK Biobank Cohort
eTable 3. Association of Average Alcohol Intake Status With Mortality in Older Drinkers From the UK Biobank Cohort, Excluding Participants With Prevalent Cancer at Baseline for Cancer Mortality, or Those With Prevalent CVD at Baseline for CVD Mortality
eTable 4. Association of Wine Preference or Drinking During Meals With Mortality in Older Drinkers From the UK Biobank Cohort, Excluding Participants With Prevalent Cancer at Baseline for Cancer Mortality, or Those With Prevalent CVD at Baseline for CVD Mortality
eTable 5. Association of Wine Preference and Drinking During Meals With Mortality in Older Drinkers From the UK Biobank Cohort, Excluding Participants With Prevalent Cancer at Baseline for Cancer Mortality, or Those With Prevalent CVD at Baseline for CVD Mortality
eTable 6. Association of Average Alcohol Intake Status With Mortality in Older Drinkers From the UK Biobank Cohort, by Drinking Patterns, Excluding Participants With Prevalent Cancer at Baseline for Cancer Mortality, or Those With Prevalent CVD at Baseline for CVD Mortality
Data Sharing Statement

See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Ortolá R , Sotos-Prieto M , García-Esquinas E , Galán I , Rodríguez-Artalejo F. Alcohol Consumption Patterns and Mortality Among Older Adults With Health-Related or Socioeconomic Risk Factors. JAMA Netw Open. 2024;7(8):e2424495. doi:10.1001/jamanetworkopen.2024.24495
Manage citations:
© 2024
- Permissions
Alcohol Consumption Patterns and Mortality Among Older Adults With Health-Related or Socioeconomic Risk Factors
- 1 Department of Preventive Medicine and Public Health, Universidad Autónoma de Madrid, Madrid, Spain
- 2 Center for Biomedical Research in Epidemiology and Public Health, Madrid, Spain
- 3 Department of Environmental Health and Nutrition, Harvard T.H. Chan School of Public Health. Boston, Massachusetts
- 4 Madrid Institute for Advanced Studies Food Institute, Campus of International Excellence Universidad Autónoma de Madrid + Spanish National Research Council, Madrid, Spain
- 5 Department of Chronic Diseases, National Center for Epidemiology, Carlos III Health Institute, Madrid, Spain
Question Do health-related or socioeconomic risk factors modify the associations of alcohol consumption patterns with mortality among older drinkers?
Findings This cohort study in 135 103 older drinkers found that even low-risk drinking was associated with higher mortality among older adults with health-related or socioeconomic risk factors. Wine preference and drinking only with meals were associated with attenuating the excess mortality associated with alcohol consumption.
Meaning This cohort study identified inequalities in the detrimental health outcomes associated with alcohol that should be addressed to reduce the high disease burden of alcohol use.
Importance Alcohol consumption is a leading cause of morbidity and mortality that may be more important in older adults with socioeconomic or health-related risk factors.
Objective To examine the association of alcohol consumption patterns with 12-year mortality and its modification by health-related or socioeconomic risk factors.
Design, Setting, and Participants This prospective cohort study used data from the UK Biobank, a population-based cohort. Participants were current drinkers aged 60 years or older. Data were analyzed from September 2023 to May 2024.
Exposure According to their mean alcohol intake in grams per day, participants’ drinking patterns were classified as occasional: ≤2.86 g/d), low risk (men: >2.86-20.00 g/d; women: >2.86-10.00 g/d), moderate risk (men: >20.00-40.00 g/d; women: >10.00-20.00 g/d) and high risk (men: >40.00 g/d; women: >20.00 g/d).
Main Outcomes and Measures Health-related risk factors were assessed with the frailty index, and socioeconomic risk factors were assessed with the Townsend deprivation index. All-cause and cause-specific mortality were obtained from death certificates held by the national registries. Analyses excluded deaths in the first 2 years of follow-up and adjusted for potential confounders, including drinking patterns and preferences.
Results A total of 135 103 participants (median [IQR] age, 64.0 [62.0-67.0] years; 67 693 [50.1%] women) were included. In the total analytical sample, compared with occasional drinking, high-risk drinking was associated with higher all-cause (hazard ratio [HR], 1.33; 95% CI, 1.24-1.42), cancer (HR, 1.39; 95% CI, 1.26-1.53), and cardiovascular (HR, 1.21; 95% CI, 1.04-1.41) mortality; moderate-risk drinking was associated with higher all-cause (HR, 1.10; 95% CI, 1.03-1.18) and cancer (HR, 1.15; 95% CI, 1.05-1.27) mortality, and low-risk drinking was associated with higher cancer mortality (HR, 1.11; 95% CI, 1.01-1.22). While no associations were found for low- or moderate-risk drinking patterns vs occasional drinking among individuals without socioeconomic or health-related risk factors, low-risk drinking was associated with higher cancer mortality (HR, 1.15; 95% CI, 1.01-1.30) and moderate-risk drinking with higher all-cause (HR, 1.10; 95% CI, 1.01-1.19) and cancer (HR, 1.19; 95% CI, 1.05-1.35) mortality among those with health-related risk factors; low-risk and moderate-risk drinking patterns were associated with higher mortality from all causes (low risk: HR, 1.14; 95% CI, 1.01-1.28; moderate risk: HR, 1.17; 95% CI, 1.03-1.32) and cancer (low risk: HR, 1.25; 95% CI, 1.04-1.50; moderate risk: HR, 1.36; 95% CI, 1.13-1.63) among those with socioeconomic risk factors. Wine preference (>80% of alcohol from wine) and drinking with meals showed small protective associations with mortality, especially from cancer, but only in drinkers with socioeconomic or health-related risk factors and was associated with attenuating the excess mortality associated with high-, moderate- and even low-risk drinking.
Conclusions and Relevance In this cohort study of older drinkers from the UK, even low-risk drinking was associated with higher mortality among older adults with health-related or socioeconomic risk factors. The attenuation of mortality observed for wine preference and drinking only during meals requires further investigation, as it may mostly reflect the effect of healthier lifestyles, slower alcohol absorption, or nonalcoholic components of beverages.
Alcohol consumption is a leading cause of morbidity and mortality, accounting for approximately 5.1% of the global burden of disease and 5.3% of all deaths and being responsible for significant social and economic losses, thus representing a major public health problem. 1 Additionally, the assumed benefits of drinking low amounts of alcohol, especially on cardiovascular disease (CVD) mortality, 2 - 4 are being questioned due to selection biases, reverse causation, and residual confounding, 5 supporting health messaging that the safest level of drinking is no drinking at all or less is better. 6 , 7 Selection biases are often overlooked, but they can lead to a systematic underestimation of alcohol-related burden. That is the case of the abstainer bias, whereby the apparently lower mortality of light drinkers compared with abstainers could be explained by the higher death risk of the abstainers because they include former drinkers who quit alcohol due to poor health, as well as lifetime abstainers, 5 who often have worse lifestyle and health characteristics than regular drinkers. 8 Also, the healthy drinker/survivor bias, caused by overrepresentation of healthier drinkers who have survived the deleterious effects of alcohol, can distort comparisons, especially in older age. 5 In addition, drinking habits may influence the association between the amount of alcohol consumed and health. In this context, wine preference has been associated with lower risk of death, 9 CVD morbimortality, 10 and diabetes, 11 attributing the beneficial associations of wine to its high content in polyphenols. 12 Furthermore, drinking with meals has been associated with lower risk of all-cause, non-CVD, and cancer deaths 13 and frailty, 14 so this might be a safer option for alcohol drinkers along with moderate consumption. 15
The health impact of alcohol consumption may be greater in individuals with socioeconomic or health-related risk factors. On one hand, older adults with health-related risk factors are more susceptible to the harmful outcomes associated with alcohol due to their greater morbidity, higher use of alcohol-interacting drugs, and reduced tolerance. 16 , 17 However, some studies have observed benefits of alcohol on unhealthy aging or frailty, especially of light alcohol intake 18 , 19 and of a Mediterranean alcohol drinking pattern, defined as moderate alcohol consumption, preferably wine and accompanying meals, 14 , 20 suggesting that the protective associations of these potentially beneficial drinking patterns might be greater in individuals with ill health, although they might be due to the aforementioned methodological issues. 5 Therefore, it would be of interest to examine whether health-related risk factors modify the associations between alcohol consumption patterns and mortality.
On the other hand, there is evidence that socioeconomically disadvantaged populations have higher rates of alcohol-related harms for equivalent and even lower amounts of alcohol, probably due to the coexistence of other health challenges, including less healthy lifestyles, and lower social support or access to health care. 21 , 22 Also, the potentially beneficial associations of wine preference and drinking during meals might be more important in individuals with socioeconomic risk factors. However, to our knowledge, no previous research has examined whether socioeconomic status modifies the associations between these potentially beneficial drinking patterns and health.
Therefore, the aim of our study is to examine the associations of several potentially beneficial alcohol consumption patterns, that is, consumption of low amounts of alcohol, wine preference, and drinking only during meals, with all-cause, cancer, and CVD mortality in older adults and their modification by health-related or socioeconomic risk factors, while addressing the main methodological issues deemed to bias such associations. Thus, we restrict analyses to current drinkers and use occasional drinkers instead of abstainers as the reference group to prevent selection biases, exclude deaths in the first 2 years of follow-up to reduce reverse causation, and adjust analyses for many sociodemographic, lifestyle, and clinical variables to palliate residual confounding. We also restrict analyses to older adults because most deaths occur in this population group, which also has a high prevalence of health-related risk factors and because the protective associations of alcohol consumption have been specifically observed in older adults, 6 which is consistent with our aim to study potentially beneficial drinking patterns.
This cohort study was approved by the North West Multi-Centre Research Ethics Committee, and all participants provided written informed consent before enrollment. This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
We used data from the UK Biobank cohort, a multicenter, prospective, population-based study with more than 500 000 participants aged 40 to 69 years identified from National Health Service primary care registers and enrolled at 22 assessment sites across England, Scotland, and Wales between 2006 and 2010. At the baseline assessment visit, they completed a computer-assisted interview and a touch-screen questionnaire on sociodemographic, lifestyle, and clinical characteristics, provided biological samples, and underwent physical and medical examinations. They were followed-up for mortality through linkage to national death registries. Additional information on the UK Biobank study has been reported elsewhere. 23 , 24
At the baseline assessment visit, study participants were asked about the frequency and mean amount of the main types of alcoholic beverages that they consumed, and alcohol content was estimated by multiplying the volume ingested (in milliliters) by the volume percentage of alcohol (4.5% for beer and cider, 11.5% for white and sparkling wine, 13% for red wine, 20% for fortified wine, and 40% for spirits) and by the specific gravity of ethanol (0.789 g/mL). According to their mean alcohol intake, drinking patterns were classified into occasional (≤2.86 g/d), low risk (men: >2.86-20.00 g/d; women: >2.86-10.00 g/d), moderate risk (men: >20.00-40.00 g/d; women: >10-20.00 g/d), and high risk (men: >40.00 g/d; women: >20.00 g/d), a categorization based on the recommendations from health authorities that we have used in previous studies. 25 - 27 When more than 80% of alcohol came from a certain type of beverage, drinkers were classified as with preference for wine, with preference for other drinks, or with no preference. 27 Participants were also classified as drinkers only during meals and as drinkers either only outside of meals or at any time. Finally, participants were classified as drinkers with no wine preference nor drinking only during meals, drinkers with wine preference or drinking only during meals, and drinkers with wine preference and drinking only during meals.
Health-related risk was assessed at baseline using the frailty index (FI) developed specifically for the UK Biobank 28 based on the procedure used by Rockwood et al. 29 A total of 49 health deficits were considered, most dichotomously (1 point if present and 0 points otherwise), and a few according to severity (0 points for no deficit, 0.25-0.75 points for mild to moderate deficits, and 1 point for severe deficit). The FI score was calculated as the total sum of points assigned to each health deficit divided by the number of deficits considered and ranged from 0.00 to 0.57. The complete list of health deficits and associated scores can be found in eTable 1 in Supplement 1 . Participants were considered to have health-related risk factors if they were prefrail or frail (FI > 0.12). 28
Socioeconomic risk was assessed at baseline using the Townsend deprivation index (TDI), 30 which measures the level of an area’s socioeconomic deprivation. TDI ranges from −6.26 to 10.16, with higher score indicating greater deprivation. Participants were considered to have socioeconomic risk factors if they lived in more deprived areas (TDI > 0) and not if they lived in more affluent areas (TDI ≤ 0).
Information on mortality was obtained from death certificates held by the National Health Service (NHS) Information Centre (NHS England) up to September 30, 2021, for participants in England and Wales, and by the NHS Central Register Scotland (National Records of Scotland) up to October 31, 2021, for participants in Scotland. 31 , 32 Length of follow-up was estimated as the time from the baseline assessment visit to the date of death or administrative censoring, whichever came first. Cause-specific mortality was ascertained with the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision ( ICD-10 ) classification 33 : codes C00 to C97 as primary cause of death for cancer and codes I00 to I99 for CVD.
We also used baseline information on sociodemographic, lifestyle, and clinical characteristics, including sex, age, self-reported race and ethnicity, education (college or university degree; A levels, AS levels, or equivalent; O levels, General Certificate of Secondary Education, or equivalent; Certificate of Secondary Education or equivalent; National Vocational Qualification, Higher National Diploma, Higher National Certificate, or equivalent; other professional qualifications; and no qualifications), tobacco smoking (never, former, or current), leisure-time physical activity (metabolic equivalents of task-hours per week), time spent watching television (hours per day), and prevalent morbidities (diabetes, CVD, and cancer) that could have a potential effect on the amount of alcohol consumed. In the UK Biobank, race and ethnicity are classified as Asian (Indian, Pakistani, Bangladeshi, any other Asian background), Black (Caribbean, African, any other Black background), Chinese, multiple (White and Black Caribbean, White and Black African, White and Asian, any other mixed background), White (British, Irish, any other White background), and other (any group not specified, eg, Arab).
From 217 462 participants aged at least 60 years in the UK Biobank cohort, we excluded 36 284 with incomplete information on alcohol consumption, 10 456 never drinkers, 8295 former drinkers, and 20 167 known binge drinkers (those who consumed ≥6 units of alcohol in 1 session) to avoid classifying binge drinkers with low mean alcohol intake as low-risk drinkers. We additionally excluded 1140 participants who died in the first 2 years of follow-up and 6017 participants with missing information on the FI (194 participants), the TDI (116 participants), and potential confounders (5707 participants). Thus, the analytical sample included 135 103 individuals.
The associations of alcohol consumption patterns (mean alcohol intake status, wine preference, and drinking during meals) at baseline with all-cause and cause-specific mortality were summarized with hazard ratios (HRs) and their 95% CIs obtained from Cox regression; the models included interactions between alcohol consumption patterns and health-related or socioeconomic risk factors and adjusted for baseline sociodemographic (sex, age, race and ethnicity, education, and TDI [except when stratifying by socioeconomic risk factors]), lifestyle (tobacco smoking, leisure-time physical activity, and time spent watching television), and clinical characteristics (diabetes, CVD, cancer, and FI score [except when stratifying by health-related risk factors]) of study participants. Analyses of alcohol intake were further adjusted for wine preference and drinking during meals, whereas analyses of wine preference and drinking during meals were further adjusted for mean alcohol intake and the other drinking pattern.
To characterize whether wine preference and drinking during meals modified the association of mean alcohol intake with mortality, we tested interaction terms defined as the product of the categories of mean alcohol intake by 3 categories of drinking patterns (no wine preference nor drinking only during meals, wine preference or drinking only during meals, and wine preference and drinking only during meals).
Additionally, we assessed whether sociodemographic and lifestyle variables modified the study associations by testing interaction terms defined as the product of alcohol consumption patterns by categories of such variables (except mean alcohol intake status by sex, as sex was included in the definition of alcohol intake status). Since no interactions were found, the results are presented for the total sample. Finally, we performed additional sensitivity analyses excluding participants with prevalent cancer at baseline for cancer mortality or those with prevalent CVD at baseline for CVD mortality.
Statistical significance was set at 2-sided P < .05. Analyses were performed with Stata software version 17 (StataCorp). Data were analyzed from September 2023 to May 2024.
A total of 135 103 participants (median [IQR] age, 64.0 [62.0-67.0] years; 67 693 [50.1%] women) were included. Occasional drinkers less often identified as White; were more frequently residents in England, women, and never smokers; were less physically active; had a lower educational level, a lower prevalence of CVD; and had a higher prevalence of diabetes, cancer, and health-related risk factors. Having socioeconomic risk factors was less frequent in low- and moderate-risk drinkers ( Table 1 ).
Over a median (range) follow-up of 12.4 (2.0 to 14.8) years, 15 833 deaths were recorded, including 7871 cancer deaths and 3215 CVD deaths. Compared with occasional drinking, low-risk drinking was associated with higher cancer mortality (HR, 1.11; 95% CI, 1.01-1.22); moderate-risk drinking was associated with higher all-cause (HR, 1.10; 95% CI, 1.03-1.18) and cancer (HR, 1.15; 95% CI, 1.05-1.27) mortality; and high-risk drinking was associated with higher all-cause (HR, 1.33; 95% CI, 1.24-1.42), cancer (HR, 1.39; 95% CI, 1.26-1.53), and CVD (HR, 1.21; 95% CI, 1.04-1.41) mortality ( Table 2 ). Hazards were greater in individuals with health-related or socioeconomic risk factors vs those without across categories of alcohol intake. Interestingly, while no associations with mortality were found in participants without health-related or socioeconomic risk factors among low- or moderate-risk drinkers, low-risk drinkers with health-related risk factors had higher cancer mortality (HR, 1.15; 95% CI, 1.01-1.30) and moderate-risk drinkers with health-related risk factors had higher all-cause (HR, 1.10; 95% CI, 1.01-1.19) and cancer (HR, 1.19; 95% CI, 1.05-1.35) mortality ( Table 2 ). Likewise, both low-risk and moderate-risk drinkers with socioeconomic risk factors showed higher mortality from all causes (low risk: HR, 1.14; 1.01-1.28; moderate risk: 1.17; 95% CI, 1.03-1.32) and cancer (low-risk: HR, 1.25; 95% CI, 1.04-1.50; moderate risk: HR, 1.36; 95% CI, 1.13-1.63) ( Table 2 ).
Wine preference and drinking only during meals were associated with lower all-cause mortality only in participants with health-related risk factors (wine preference: HR, 0.92; 95% CI, 0.87-0.97; drinking only during meals: HR, 0.93; 95% CI, 0.89-0.97), as well as in participants with socioeconomic risk factors (wine preference: HR, 0.84; 95% CI, 0.78-0.90; drinking only during meals: HR, 0.83; 95% CI, 0.78-0.89) ( Table 3 ). Drinking only during meals was also associated with lower cancer mortality in participants with health-related risk factors (HR, 0.92; 95% CI, 0.86-0.99) or socioeconomic risk factors (HR, 0.85; 95% CI, 0.78-0.94) ( Table 3 ). Furthermore, in individuals with socioeconomic risk factors, wine preference was associated with lower cancer mortality (HR, 0.89; 95% CI, 0.80-0.99) and drinking only during meals with lower CVD mortality (HR, 0.86; 95% CI, 0.75-1.00) ( Table 3 ). Adhering to both drinking patterns was associated with lower all-cause, cancer, and CVD mortality in drinkers with health-related or socioeconomic risk factors, and to a lesser extent, with lower all-cause death in drinkers without health-related risk factors (eTable 2 in Supplement 1 ). Importantly, wine preference and drinking during meals modified the association of mean alcohol intake with mortality: the excess risk of all-cause, cancer, and CVD death for high-risk drinkers, of all-cause and cancer death for moderate-risk drinkers, and of cancer death for low-risk drinkers vs occasional drinkers was attenuated and even lost among individuals with these drinking patterns ( Table 4 ). Analyses excluding participants with prevalent cancer at baseline for cancer mortality, or those with prevalent CVD at baseline for CVD mortality showed consistent results (eTables 3-6 in Supplement 1 ).
This cohort study in older alcohol drinkers from the UK found that compared with occasional drinkers, low-risk drinkers had higher cancer mortality, moderate-risk drinkers had higher all-cause and cancer mortality, and high-risk drinkers had higher all-cause, cancer, and CVD mortality. The excess mortality associated with alcohol consumption was higher in individuals with health-related and socioeconomic risk factors, among whom even low-risk drinkers had higher mortality, especially from cancer. Wine preference and drinking only with meals showed small protective associations with mortality, especially from cancer, among drinkers with health-related and socioeconomic risk factors, and these 2 drinking patterns attenuated the excess mortality associated with high-, moderate-, and even low-risk drinking.
In line with recent research on the associations between alcohol use and health, 6 , 34 , 35 our results corroborate the detrimental outcomes associated with heavy drinking in older adults. However, we also found higher risk for all-cause and cancer deaths in moderate-risk drinkers, unlike most previous research, which has reported protective associations of low to moderate alcohol consumption, mainly for all-cause 2 - 4 , 36 and CVD 3 , 36 , 37 mortality, ischemic heart disease, 3 , 6 , 34 and diabetes, 6 or null associations with all-cause mortality, 38 CVD, 39 and unhealthy aging. 20 This discrepancy may be due to the implementation of an important methodological improvement in our analyses, that is, using occasional drinkers as the reference group instead of lifetime abstainers, to prevent selection bias caused by misclassification of former drinkers as abstainers, and to palliate residual confounding because they are more like light drinkers than are never drinkers. 40 , 41 In fact, another analysis of the UK Biobank cohort that also avoided selection biases found an increased CVD risk in the general population for drinking up to 14 units per week. 42
To our knowledge, there are no studies examining the potential modification of health-related risk factors on the association between alcohol use and health. The stronger associations between mean alcohol intake and mortality observed in older adults with health-related risk factors make sense, since they have more morbid conditions potentially aggravated by alcohol and greater use of alcohol-interacting medications than their counterparts without health-related risk factors. 16 , 17 The fact that even low-risk drinkers with these risk factors had higher risk of cancer death is an important finding, which is consistent with the reported increased risk of several types of cancer and cancer mortality even with very low amounts of alcohol. 6 , 36 , 37 , 43
Our results also suggest that socioeconomic status acts as a modifier of the association between the amount of alcohol consumed and mortality, as mortality hazard was much greater in individuals with socioeconomic risk factors than in individuals without, in line with previous research. 21 , 22 , 44 , 45 We even found a detrimental association of low amounts of alcohol with all-cause and cancer mortality in this group, unlike the MORGAM study by DiCasetnuovo et al 44 reporting a lower mortality associated with consuming no more than 10 g/d of alcohol, which was clearer in individuals with higher vs lower education. 44 These discrepant results could again be explained by the different reference groups used: occasional drinkers in our study and never drinkers in the MORGAM study. Importantly, although older adults with socioeconomic risk factors have a higher risk of ill health and death, probably due to the coexistence of other health challenges, especially poorer lifestyles, 21 , 22 the observed associations in our study were independent of lifestyles, suggesting that other factors should account for them.
Regarding the potentially beneficial drinking patterns, that is, wine preference and drinking during meals, the literature is inconsistent. A 2018 pool of studies 34 reported a nondifferential association of specific types of alcoholic drinks with all-cause mortality and several CVD outcomes, whereas other studies have found protective health associations for wine but not other beverages. 15 , 46 Drinking with meals has also shown protective associations with several health outcomes. 15 In our analysis, these drinking patterns modified the association between alcohol intake and death risk. On one hand, the protective association for mortality of these patterns was only observed in individuals with socioeconomic or health-related risk factors, independently of the amount of alcohol consumed. On the other hand, the detrimental association of alcohol intake was more evident in individuals without these patterns. These findings suggest that the less detrimental associations of alcohol intake from wine or during meals are not due to alcohol itself, but to other factors, including nonalcoholic components of wine, such as antioxidants, slower absorption of alcohol ingested with meals and its consequent reduced alcoholaemia, as well as spacing drinks when drinking only with meals, or more moderate attitudes in individuals who choose to adhere to these drinking patterns.
Our study has several strengths, such as the large sample size, the long follow-up, and the methodological improvements implemented to prevent selection biases and reduce reverse causation. However, it also has some limitations. First, alcohol intake was self-reported, and therefore prone to some degree of misclassification. Also, alcohol intake was measured only at baseline and not at multiple time points over the life span, not allowing us to take into account changes in alcohol intake before the baseline assessment or to redistribute former drinkers among categories of current drinkers to reduce selection bias; this may have led to an underestimation of the true effects of alcohol consumption. 5 Second, as in any observational study, we cannot entirely rule out residual confounding, despite adjusting for many potential confounders. And third, this study was conducted in older adults in the UK with a high proportion of White participants, so our results may not be generalizable to other racial ethnic groups or populations with different lifestyles, drinking patterns, or socioeconomic development.
This cohort study among older drinkers from the UK did not find evidence of a beneficial association between low-risk alcohol consumption and mortality; however, we observed a detrimental association of even low-risk drinking in individuals with socioeconomic or health-related risk factors, especially for cancer deaths. The attenuation of the excess mortality associated with alcohol among individuals who preferred to drink wine or drink only during meals requires further investigation to elucidate the factors that may explain it. Finally, these results have important public health implications because they identify inequalities in the detrimental health outcomes associated with alcohol that should be addressed to reduce the high burden of disease of alcohol use.
Accepted for Publication: May 30, 2024.
Published: August 12, 2024. doi:10.1001/jamanetworkopen.2024.24495
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Ortolá R et al. JAMA Network Open .
Corresponding Author: Rosario Ortolá, MD, PhD, Department of Preventive Medicine and Public Health, School of Medicine, Universidad Autónoma de Madrid, Calle del Arzobispo Morcillo 4, 28029 Madrid, Spain ( [email protected] ).
Author Contributions: Dr Ortolá had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Ortolá.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Ortolá.
Critical review of the manuscript for important intellectual content: Sotos-Prieto, García-Esquinas, Galán, Rodríguez-Artalejo.
Statistical analysis: Ortolá.
Obtained funding: Sotos-Prieto, Rodríguez-Artalejo.
Administrative, technical, or material support: Rodríguez-Artalejo.
Supervision: García-Esquinas, Galán.
Conflict of Interest Disclosures: None reported.
Funding/Support: This work was supported by the Plan Nacional sobre Drogas, Ministry of Health of Spain (grant No. 2020/17), Instituto de Salud Carlos III, State Secretary of R+D+I and Fondo Europeo de Desarrollo Regional/Fondo Social Europeo (Fondo de Investigación en Salud grants No. 19/319, 20/896, and 22/1111), Agencia Estatal de Investigación (grant No. CNS2022-135623), Carlos III Health Institute and the European Union “NextGenerationEU (grant No. PMP21/00093), and the Fundación Francisco Soria Melguizo (Papel de la Disfunción Mitocondrial en la Relación Entre Multimorbilidad Crónica y Deterioro Funcional en Ancianos project grant). Mercedes Sotos-Prieto holds a Ramón y Cajal contract (contract No. RYC-2018-025069-I) from the Ministry of Science, Innovation and Universities.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Systematic Review
- Open access
- Published: 25 August 2022
Age-related differences in the effect of chronic alcohol on cognition and the brain: a systematic review
- Lauren Kuhns ORCID: orcid.org/0000-0002-3156-8905 1 , 2 ,
- Emese Kroon ORCID: orcid.org/0000-0003-1803-9336 1 , 2 ,
- Heidi Lesscher 3 ,
- Gabry Mies 1 &
- Janna Cousijn 1 , 2 , 4
Translational Psychiatry volume 12 , Article number: 345 ( 2022 ) Cite this article
5201 Accesses
8 Citations
3 Altmetric
Metrics details
- Human behaviour
Adolescence is an important developmental period associated with increased risk for excessive alcohol use, but also high rates of recovery from alcohol use-related problems, suggesting potential resilience to long-term effects compared to adults. The aim of this systematic review is to evaluate the current evidence for a moderating role of age on the impact of chronic alcohol exposure on the brain and cognition. We searched Medline, PsycInfo, and Cochrane Library databases up to February 3, 2021. All human and animal studies that directly tested whether the relationship between chronic alcohol exposure and neurocognitive outcomes differs between adolescents and adults were included. Study characteristics and results of age-related analyses were extracted into reference tables and results were separately narratively synthesized for each cognitive and brain-related outcome. The evidence strength for age-related differences varies across outcomes. Human evidence is largely missing, but animal research provides limited but consistent evidence of heightened adolescent sensitivity to chronic alcohol’s effects on several outcomes, including conditioned aversion, dopaminergic transmission in reward-related regions, neurodegeneration, and neurogenesis. At the same time, there is limited evidence for adolescent resilience to chronic alcohol-induced impairments in the domain of cognitive flexibility, warranting future studies investigating the potential mechanisms underlying adolescent risk and resilience to the effects of alcohol. The available evidence from mostly animal studies indicates adolescents are both more vulnerable and potentially more resilient to chronic alcohol effects on specific brain and cognitive outcomes. More human research directly comparing adolescents and adults is needed despite the methodological constraints. Parallel translational animal models can aid in the causal interpretation of observed effects. To improve their translational value, future animal studies should aim to use voluntary self-administration paradigms and incorporate individual differences and environmental context to better model human drinking behavior.
Similar content being viewed by others
Systematic review and meta-analysis on the effects of chronic peri-adolescent cannabinoid exposure on schizophrenia-like behaviour in rodents
Yohimbine as a pharmacological probe for alcohol research: a systematic review of rodent and human studies

Consequences of adolescent drug use
Introduction.
Alcohol use disorder (AUD) is the most prevalent substance use disorder worldwide [ 1 ]. Most AUDs remain untreated [ 2 ] and for those seeking treatment, relapse rates are high [ 3 ]. Adolescence marks a rapid increase in AUD and an earlier onset of AUD is associated with worse long-term outcomes, including greater problem severity and more relapses [ 4 , 5 ]. Loss of control over alcohol use is a core aspect of AUD [ 6 ] and the developmentally normative difficulty to control motivational urges in tempting and arousing situations is thought to put adolescents at risk for developing addictive behaviors [ 7 ]. Moreover, neurotoxic consequences of alcohol use may be more severe for a developing brain [ 8 ]. Paradoxically, adolescence is also a period of remarkable behavioral flexibility and neural plasticity [ 9 , 10 , 11 ], allowing adolescents to adapt their goals and behavior to changing situations [ 12 ] and to recover from brain trauma more easily than adults [ 10 ]. In line with this, the transition from adolescence to adulthood is associated with high rates of AUD recovery without formal intervention [ 13 ]. While the adolescent brain may be a vulnerability for the development of addiction, it may also be more resilient to long-term effects compared to adults. Increased neural plasticity during this period could help protect adolescents from longer-term alcohol use-related cognitive impairments across multiple domains, from learning and memory to decision-making and cognitive flexibility. Therefore, the goal of this systematic review was to examine the evidence of age-related differences in the effect of alcohol on the brain and cognitive outcomes, evaluating evidence from both human and animal studies.
In humans, the salience and reinforcement learning network as well as the central executive network are involved in the development and maintenance of AUD [ 7 , 14 ]. The central executive network encompasses fronto-parietal regions and is the main network involved in cognitive control [ 15 ]. The salience network encompasses fronto-limbic regions crucial for emotion regulation, salience attribution, and integration of affective information into decision-making [ 15 , 16 ], which overlaps with fronto-limbic areas of the reinforcement learning network (Fig. 1 ). Relatively early maturation of salience and reinforcement learning networks compared to the central executive network is believed to put adolescents at heightened risk for escalation of alcohol use compared to adults [ 7 ]. Rodent models are regularly used for AUD research and allow in-depth neurobehavioral analyses of the effects of ethanol exposure during different developmental periods while controlling for experimental conditions such as cumulative ethanol exposure in a way that is not possible using human subjects because exposure is inherently confounded with age. For example, animal models allow for detailed neurobiological investigation of the effects of alcohol exposure in a specific age range on neural activation, protein expression, gene expression, epigenetic changes, and neurotransmission in brain regions that are homologous to those that have been implicated in AUD in humans.
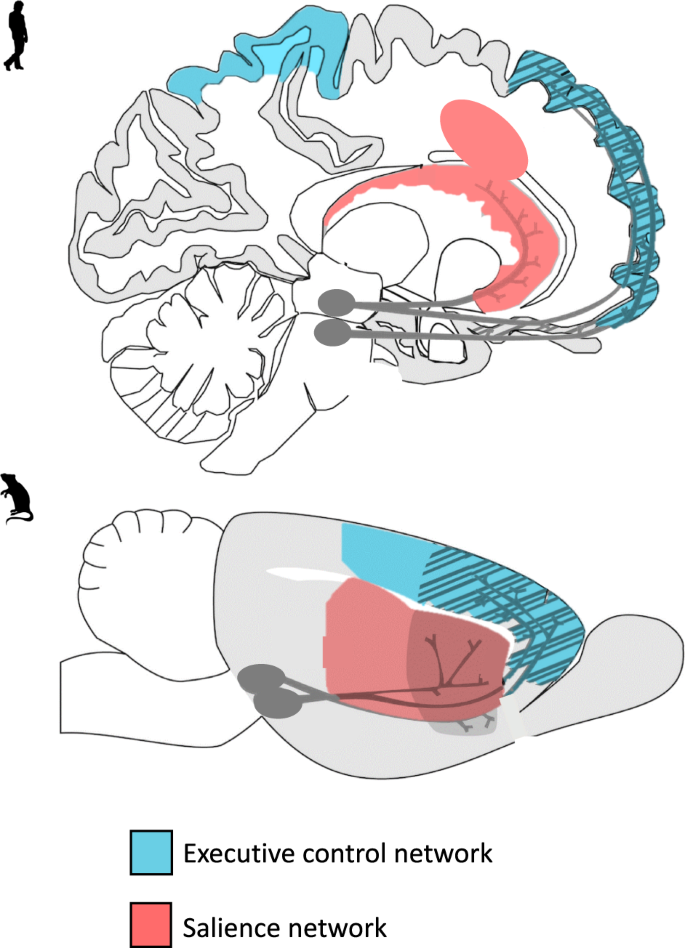
A visual representation of the translational model of the executive control and salience networks in humans and rodents. The executive control and salience are key networks believed to play a part in adolescent vulnerability to alcohol-related problems.
While most of our knowledge on the effects of alcohol on the brain and cognitive outcomes is based on research in adults, several recent reviews have examined the effects of alcohol on the brain and cognition in adolescents and young adults specifically [ 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 ]. Heavy or binge drinking has been associated with reduced gray and white matter. Also, altered task-related brain activity [ 20 ], structural abnormalities [ 25 ], and overlapping behavioral impairment in executive functioning have been identified in adolescent and young adult alcohol users [ 19 ]. While some of the observed neurocognitive differences between drinkers and non-drinkers may be predisposing factors, they may be further exacerbated by heavy and binge drinking [ 21 , 23 ]. Furthermore, reviews of longitudinal studies concluded that adolescent alcohol use is associated with neural and cognitive alterations in a dose-dependent manner [ 17 , 22 ].
Although previous reviews underscore the potential negative consequences of heavy alcohol use on the brain and cognition in adolescence, they do not typically address the question of whether adolescents are differentially vulnerable compared to adults to the effects of alcohol on these outcomes. Explicit comparisons between adolescents and adults are crucial to identify potential risk and resilience factors. In the current review, we aimed to extend previous work by systematically examining this critical question: does the relationship between chronic alcohol use and neurocognitive outcomes differ between adolescents and adults? To address this question, we systematically reviewed human and animal studies that included both age groups and used a factorial design that would allow for the comparison of the effects of chronic alcohol use on cognitive and brain-related outcomes across age groups. We specifically highlight outcomes from voluntary self-administration paradigms when available and discuss the translational quality of the animal evidence base. We conclude with a discussion of prominent knowledge gaps, future research directions, and clinical implications.
Study inclusion criteria and search strategy
We followed the PRISMA guidelines for the current systematic review (The PRIMSA Group, 2009). An initial MedLine, Cochrane Library, and PsycInfo search was conducted during September of 2018 with terms related to alcohol, cognition, adolescence/adulthood, and study type (see Appendix for full search strategy and syntax). Two search updates using the same search strategy were conducted on 31 March 2020 and 3 February 2021. For all searches, the identified citations were split into batches and at least two of the following assessors (GM, LK, JC, or CG) conducted a blinded review to determine whether articles met the inclusion criteria. In the first phase of screening, only titles and abstracts were screened and articles that clearly did not meet the inclusion criteria were excluded. In the second phase, the remaining articles received a full-text review and those that did not meet all inclusion criteria were excluded. The first inclusion criterion that was not adhered to was recorded as the reason for excluding. If there was a discrepancy between authors after initial and full-text screening process, the reviewing authors discussed the article and a consensus was reached.
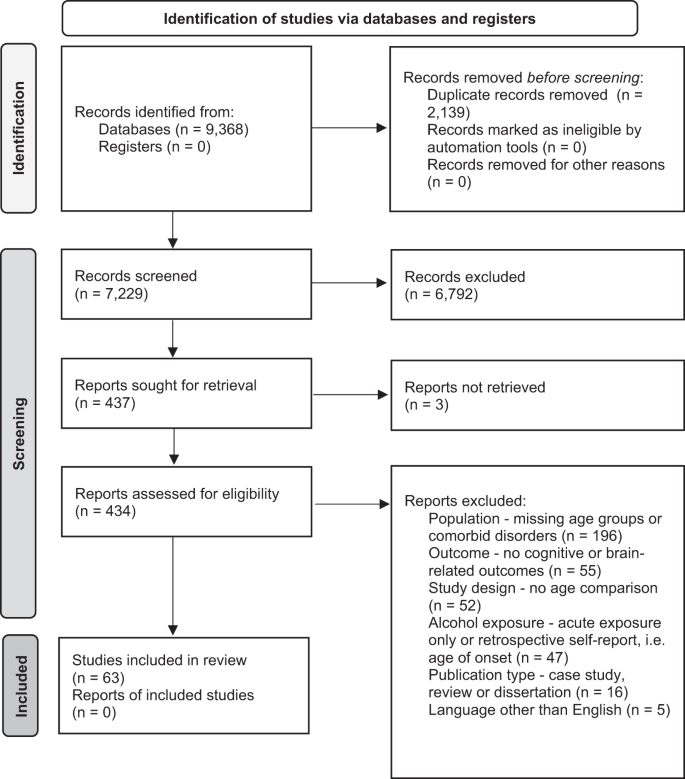
The inclusion criteria were: (1) Human samples including both adolescents younger than 18 and adults older than 18 and animal samples including adolescent (Post Natal Day (PND) 25–42 for rodents) and adult [ 8 ] animals (greater than PND 65 for rodents); (2) Exploration of alcohol as the independent variable and cognitive, reward-related, or brain outcomes as the dependent variables; (3) Alcohol and cognitive outcomes must meet our operationalization defined below; (4) Study design comparing adults and adolescents on outcome measures; (5) Administering or measuring alcohol use during adolescence or adulthood, not retrospectively (e.g., no age of onset work in humans using retrospective self-reports of alcohol consumption); (6) Primary quantitative data collection (no case studies, or review papers); (7) Solely looking at alcohol-related factors as the independent variables (e.g., cannot explore alcohol-related factors in individuals with psychosis); (8) Written in English; (9) Published in a peer-reviewed journal before February 3, 2021 (see Fig. 2 for a detailed screening process).
The definitions for adolescence are variable, hampering the direct comparison of human and rodent research. In rodents, the end of early-mid adolescence is considered to be approximately PND 42 when rats reach sexual puberty. By contrast, the boundaries for the onset of early adolescence are less clear. Based on the notion that most age-typical physiological changes that are characteristic of adolescence emerge from PND 28 [ 26 ], the conservative boundary for adolescence has been set at PND 28 (e.g., seminal review on adolescence [ 27 ]). The preceding week (PND 21-PND 28) has been described as the juvenile period (e.g., [ 28 , 29 ]) but these same reports consider PND 21-PND 23 as the lower boundary for early adolescence [ 28 , 29 ], further emphasizing that the boundary of PND28 may be too conservative. Indeed, multiple studies (e.g., [ 30 , 31 ]), have chosen to take PND25 as the boundary for early adolescence. Hence, we have decided to also follow this less conservative approach and include all studies where alcohol was administered between PND 25 and PND 42.
The exact boundaries of human adolescence are similarly nebulous. From a neurodevelopmental perspective, adolescence is now often thought of as continuing until approximately age 25 because of the continuing maturation of the brain [ 32 ]. However, the delineation of adolescence and adulthood is also dependent on societal norms, and is commonly defined as the transitional period between puberty and legal adulthood and independence which typically begins around age eighteen. In light of this, we chose a relatively liberal inclusion criteria for the human studies; studies needed to include at least some adolescents below eighteen, the age at which drinking typically begins, as well as ‘adult’ participants over the age of eighteen. We are careful to interpret the results of human studies within the neurodevelopmental framework of adolescence, such that 18–25-year-olds are considered late adolescents to young adults who are still undergoing cognitive and brain maturation.
Notably, we excluded studies that assessed alcohol exposure retrospectively (primarily early onset alcohol studies) because age of onset variables are often inaccurate, with reported age of alcohol onset increasing with both historical age [ 33 ] and current alcohol use patterns [ 34 ]. In addition, we excluded work that has not undergone peer-review to ensure high-quality papers.
In humans, we defined cognition as any construct that typically falls within the umbrella of neuropsychological testing, as well as brain-based studies. We also included more distal constructs of cognition, like craving and impulsivity, because they play a prominent role in addictive behaviors [ 35 , 36 ]. In rodents, we defined cognition as attention, learning, and memory in line with a seminal review paper [ 37 ]. Given the importance of social cognition in patterns of alcohol use particularly in adolescence [ 38 ] and its proposed role in adolescent risk and resilience to addiction [ 39 ], we included social behavior as an outcome. Furthermore, because many rodent studies assessed anxiety-related behaviors and the high degree of comorbidity between anxiety disorders and alcohol addiction [ 40 ], we also included anxiety as a secondary outcome. On the other hand, locomotor activity was excluded as an outcome because even though behavioral sensitization is considered to reflect neurobiological changes that may underlie certain aspects of addictive behavior [ 36 ], the translational relevance for addictive behavior and human addiction in particular remains unclear [ 41 , 42 ]. Across both rodents and humans, general alcohol metabolization and ethanol withdrawal studies were not included except if they included brain-related outcomes. The relevant reported findings (i.e., the results of an analysis of comparing age groups on the effect of alcohol on an included outcome) were extracted by a one reviewer and then confirmed by at least one other reviewer. In addition, the characteristics of the sample, details of alcohol exposure, and study design were extracted by a single reviewer and then confirmed by at least one other reviewer. No automation tools were used for extraction. Within the included studies, peripheral findings that did not relate to cognition were excluded from review and not extracted. The protocol for this systematic review was not registered and no review protocol can be accessed.
Study search
Our searches identified 7229 studies once duplicates were removed. A total of 6791 studies were excluded after initial review of abstracts. Then, 434 studies received a full-text review and 371 were excluded for failing to meet all inclusion criteria. See Fig. 2 for a flow diagram of the full screening process. At the end of the inclusion process, 59 rodent studies and 4 human studies were included. The characteristics and findings of the final studies are detailed in Table 1 (rodents) and Table 2 (humans). Due to the heterogeneity of outcomes, meta-regression was not suitable for synthesizing results. Results are narratively synthesized and grouped based on forced or voluntary ethanol exposure and by outcome within the tables and by outcome only in text. Two authors independently rated the quality of evidence for human studies (Table 2 ) based on criteria used in a similar systematic review [ 43 ]: (1) strong level of causality: longitudinal design comparing adolescent and adults while adjusting for relevant covariates; (2) moderate level of causality: longitudinal design comparing adolescents and adults without adjusting for relevant covariates or cross-sectional designs with matched groups that considered relevant covariates; (3) weak level of causality: cross-sectional design without matched adolescent and adult groups and/or did not adjust for relevant covariates. A methodological quality assessment was not conducted for the animal studies due to a lack of empirically validated risk of bias tools and lack of standardized reporting requirements in the animal literature.
PRIMSA flow diagram detailing the screening process.
Animal studies
Cognitive outcomes, learning and memory.
Human evidence clearly suggests that alcohol is related to learning and memory impairments, both during intoxication [ 44 ] and after sustained heavy use and dependence [ 45 , 46 ]. Paradigms that assess learning and memory provide insight into the negative consequences of alcohol consumption on brain functioning, as well as the processes underlying the development and maintenance of learned addictive behaviors.
Conditioned alcohol aversion or preference: Lower sensitivity to alcohol’s aversive effects (e.g., nausea, drowsiness, motor incoordination) but higher sensitivity to alcohol’s rewarding effects has been hypothesized to underlie the higher levels of alcohol use, especially binge-like behavior, in adolescents compared to adults [ 47 ]. Several conditioning paradigms have been developed to assess the aversive and motivational effects of alcohol exposure.
The conditioned taste aversion (CTA) paradigm is widely used to measure perceived aversiveness of alcohol in animals. Repeated high-dose ethanol injections are paired with a conditioned stimulus (CS, e.g., a saccharin or NaCL solution). The reduction in CS consumption after conditioning is used as an index of alcohol aversion. Two studies examined CTA in mice [ 48 , 49 ] and two in rats [ 50 , 51 ]. Three of the four studies found age-related differences. In all three studies using a standard CTA paradigm, adolescents required a higher ethanol dosage to develop aversion compared to adults [ 48 , 49 , 50 ]. Using a similar second-order conditioning (SOC) paradigm pairing high doses of ethanol (3.0 g/kg) with sucrose (CS), both adolescent and adult rats developed equal aversion to the testing compartment paired with ethanol [ 51 ].
Overall, three studies found support for lower sensitivity to alcohol’s aversive effects in adolescents, whereas one observed no differences. Future research should employ intragastric as opposed intraperitoneal exposure to better mimic human binge-like drinking in order to increase the translational value of the findings.
To measure differences in alcohol’s motivational value, conditioned place preference (CPP) paradigms have been used. This involves repeated pairings of ethanol injections with one compartment and saline injections with another compartment of the testing apparatus. On test days, CPP is assessed by measuring how long the animal stays in the compartment paired with ethanol relative to saline injections. Four studies examined CPP, with two studies observing age-related differences [ 52 , 53 , 54 , 55 ]. In the only mouse study, history of chronic ethanol exposure during adolescence (2.0 g/kg for 15 days) but not adulthood [ 52 ] led to increased CPP after brief abstinence (5 days) before the conditioning procedure (2.0 g/kg, four doses over 8 days). This suggests that early ethanol exposure increases alcohol’s rewarding properties later on. However, two rat studies did not observe either preference or aversion in either age when using lower ethanol doses and a shorter exposure period (0.5 and 1.0 g/kg for 8 days) [ 53 ], nor when using higher doses and intermittent exposure (3.0 g/kg, 2 days on, 2 days off schedule) [ 55 ]. Next to species and exposure-specific factors, environmental factors also play a role [ 54 ], with adolescents raised in environmentally enriched conditions demonstrating CPP (2 g/kg) while adolescents raised in standard conditions did not. In contrast, CPP was insensitive to rearing conditions in adults with both enriched and standard-housed rats showing similar levels of CPP.
Overall, there is inconsistent evidence for age-related differences in the motivational value of ethanol. One study found support for increased sensitivity to the rewarding effects of ethanol in adolescents, whereas one found support for adults being more sensitive and two observed no differences.
Fear conditioning and retention: Pavlovian fear conditioning paradigms are used to investigate associative learning and memory in animals. These paradigms are relevant for addiction because fear and drug-seeking behavior are considered conditioned responses with overlapping neural mechanisms [ 56 ]. Rodents are administered an unconditioned stimulus (US; e.g., foot shock) in the presence of a conditioned stimulus (CS; unique context or cue). Conditioned responses (CR; e.g., freezing behavior) are then measured in the presence of the CS without the US as a measure of fear retention. Contextual fear conditioning is linked to hippocampus and amygdala functioning and discrete cue-based (e.g., tone) fear is linked to amygdala functioning. [ 57 , 58 , 59 ], and fear extinction involves medial PFC functioning [ 60 ]. Five studies investigated fear conditioning, four in rats [ 61 , 62 , 63 , 64 ] and one in mice [ 65 ].
Only one of the four studies observed age-related differences in tone fear conditioning. Bergstrom et al. [ 61 ] found evidence for impaired tone fear conditioning in male and female alcohol-exposed (18d) adolescent compared to adult rats after extended abstinence (30d). However, adolescent rats consumed more ethanol during the one-hour access period than adults, which may explain the observed age differences in fear tone conditioning. Small but significant sex differences in consumption also emerged in the adolescent group, with males showing more persistent impairment across the test sessions compared to females, despite adolescent females consuming more ethanol than males. In contrast, three studies found no evidence of impaired tone fear conditioning in either age group after chronic alcohol exposure (4 g/kg, every other day for 20d) and extended abstinence [ 62 , 63 ] (22d), [ 64 ].
Two of the three studies observed age-related differences in contextual fear conditioning [ 62 , 63 , 64 ]. In two studies with similar exposure paradigms, only adolescents exposed to chronic high dosages of ethanol (4 g/kg) showed disrupted contextual fear conditioning after extended abstinence (22d) [ 62 , 63 ]. Importantly, differences disappeared when the context was also paired with a tone, which is suggestive of a potential disruption in hippocampal-linked contextual fear conditioning specifically [ 64 ]. Furthermore, there may be distinct vulnerability periods during adolescence as contextual fear retention was disrupted after chronic alcohol exposure (4 g/kg, every other day for 20d) during early-mid adolescence but not late adolescence [ 62 ]. In the only study to combine chronic exposure and acute ethanol challenges, contextual conditioning was impaired by the acute challenge (1 g/kg) but there was no effect of pre-exposure history in either age group (4 g/kg, every other day for 20d) [ 63 ].
Only one study examined fear extinction, and found no effect of ethanol exposure (4/kg, every other day for 20d) on extinction after tone conditioning. However, adults had higher levels of contextual fear extinction compared to mid-adolescents while late adolescents performed similar to adults [ 62 ]. Moreover, looking at binge-like exposure in mice (three binges, 3d abstinence), Lacaille et al. [ 65 ] showed comparable impairments in long-term fear memory in adolescents and adults during a passive avoidance task in which one compartment of the testing apparatus was paired with a foot shock once and avoidance of this chamber after a 24 h delay was measured.
In sum, there is limited but fairly consistent evidence for adolescent-specific impairments in hippocampal-linked contextual fear conditioning across two rat studies, while no age differences emerged in context-based fear retention in one study of mice. In contrast, only one of the four studies found evidence of impaired tone fear conditioning in adolescents (that also consumed more alcohol), with most finding no effect of alcohol on tone fear conditioning regardless of age. With only one study examining medial PFC-linked fear extinction, no strong conclusions can be drawn, but initial evidence suggests context-based fear extinction may be diminished in mid-adolescents compared to adults and late adolescents. Research on age-related differences on the effect of alcohol on longer-term fear memory is largely missing.
Spatial learning and memory: The Morris Water Maze (MWM) is commonly used to test spatial learning and memory in rodents. Across trials, time to find the hidden platform in a round swimming pool is used as a measure of spatial learning. Spatial memory can be tested by removing the platform and measuring the time the animal spends in the quadrant where the escape used to be. The sand box maze (SBM) is a similar paradigm in which animals need to locate a buried appetitive reinforcer.
Six rat studies examined spatial learning and memory using these paradigms. Three of the six studies observed age-related differences. Four examined the effects of repeated ethanol challenges 30 minutes prior to MWM training, showing mixed results [ 30 , 66 , 67 , 68 ]. While one found ethanol-induced spatial learning impairments in adolescents only (1.0 and 2.0 g/kg doses) [ 66 ], another found no age-related differences, with both age groups showing impairments after moderate doses (2.5 g/kg) and enhancements in learning after very low doses (0.5 g/kg) [ 67 ]. Sircar and Sircar [ 68 ] also found evidence of ethanol-induced spatial learning and memory impairments in both ages (2.0 g/kg). However, memory impairments recovered after extended abstinence (25d) in adults only. Importantly, MWM findings could be related to thigmotaxis, an anxiety-related tendency to stay close to the walls of the maze. Developmental differences in stress sensitivity may potentially confound ethanol-related age effects in these paradigms. Using the less stress-inducing SBM, adults showed greater impairments in spatial learning compared to adolescents after 1.5 g/kg ethanol doses 30 min prior to training [ 30 ].
Two studies examined the effects of chronic ethanol exposure prior to training with or without acute challenges [ 69 , 70 ]. Matthews et al. [ 70 ] looked at the effect of 20 days binge-like (every other day) pre-exposure and found no effect on spatial learning in either age following an extended abstinence period (i.e., 6–8 weeks). Swartzwelder et al. [ 69 ] examined effects of 5-day ethanol pre-exposure with and without ethanol challenges before MWM training. Ethanol challenges (2.0 g/kg) impaired learning in both age groups regardless of pre-exposure history. Thigmotaxis was also increased in both age groups after acute challenges while pre-exposure increased it in adults only.
In sum, evidence for impaired spatial learning and memory after acute challenges is mixed across six studies. Two studies found support for ethanol having a larger impact in adolescents compared to adults, whereas one study found the opposite and three studies did not observe any differences. Differences in ethanol doses stress responses may partially explain the discrepancies across studies. Importantly, given the sparsity of studies addressing the effects of long-term and voluntary ethanol exposure, no conclusion can be drawn about the impact of age on the relation between chronic alcohol exposure and spatial learning and memory.
Non-spatial learning and memory: Non-spatial learning can also be assessed in the MWM and SBM by marking the target location with a pole and moving it across trials, measuring time and distances traveled to locate the target. By assessing non-spatial learning as well, studies can determine whether learning is more generally impaired by ethanol or whether it is specific to hippocampal-dependent spatial learning processes. A total of six studies assessed facets of non-spatial learning and memory. Two of the six studies observed age-related differences.
In the four studies that examined non-spatial memory using the MWM or SBM in rats, none found an effect of alcohol regardless of dose, duration, or abstinence period in either age group [ 30 , 66 , 67 , 70 ]. Two other studies examined other facets of non-spatial memory in rats [ 65 , 71 ]. Galaj et al. [ 71 ] used an incentive learning paradigm to examine conditioned reward responses and approach behavior towards alcohol after chronic intermittent ethanol (CIE; 4 g/kg; 3d on, 2d off) exposure to mimic binge drinking. To examine reward-related learning and approach behavior, a CS (light) was paired with food pellets and approach behavior to CS only presentation and responses to a lever producing the CS were measured. In both adolescents and adults, the ethanol-exposed rats showed impaired reward-related learning after both short (2d) and extended (21d) abstinence. No effect of alcohol on conditioned approach behavior was observed in either age group during acute (2d) or extended (21d) abstinence. Using a novel object recognition test in mice, Lacaille et al. [ 65 ] assessed non-spatial recognition memory by replacing a familiar object with a novel object in the testing environment. Explorative behavior of the new object was used as an index of recognition. After chronic binge-like exposure (three injections daily at 2 h intervals) and limited abstinence (4d), only adolescents showed reduced object recognition.
Across facets of non-spatial memory, there is little evidence for age-related differences in the effect of chronic alcohol, with four of the six studies finding no age differences. For memory of visually cued target locations in the MWM and SBM paradigms, alcohol does not alter performance in either age. Also, both adolescents and adults appear similarly vulnerable to alcohol-induced impairments in reward-related learning based on the one study. Only in the domain of object memory did any age-related differences emerge, with adolescents and not adults showing reduced novel object recognition after binge-like alcohol exposure in one study. However, more research into object recognition memory and reward-related learning and memory is needed to draw strong conclusions in these domains.
Executive function and higher-order cognition
Executive functions are a domain of cognitive processes underlying higher-order cognitive functions such as goal-directed behavior. Executive functions can include but are not limited to working memory, attentional processes, cognitive flexibility, and impulse control or inhibition [ 72 ]. A core feature of AUD is the transition from goal-directed alcohol use to habitual, uncontrolled alcohol use. Impaired executive functioning, linked to PFC dysfunction [ 73 ], is assumed to be both a risk factor and consequence of chronic alcohol use. A meta-analysis of 62 studies highlighted widespread impairments in executive functioning in individuals with AUD that persisted even after 1-year of abstinence [ 46 ]. Thirteen studies examined facets of executive functioning and higher-order cognition, specifically in the domains of working memory, attentional processes, cognitive flexibility, impulsivity in decision-making, and goal-directed behavior [ 65 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 ].
Working memory: Working memory refers to the limited capacity system for temporarily storing and manipulating information, which is necessary for reasoning and decision-making [ 84 ]. In the Radial Arm Maze test (RAM) [ 85 ], some of the equally spaced arms (typically eight) around a circular platform contain a food reward for animals to find. Spatial working memory is measured by recording the number of revisits to previously visited arms (i.e., working memory error) and first entries into unbaited arms (i.e., reference memory). Alternatively, the hippocampus mediated [ 86 ] spontaneous tendency to alternate arms can be used as a measure of spatial working memory. In this case, revisiting an arm in back-to-back trials in close temporal succession is interpreted as a working memory error. Five studies examined the effects of chronic ethanol exposure on spatial working memory [ 65 , 75 , 79 , 80 , 83 ]. One of the five studies observed age-related differences.
Chronic binge-like alcohol exposure had no effects on spontaneous alterations after prolonged abstinence (2d on, 2d off; 3 weeks abstinence) [ 79 , 80 ] in rats or limited abstinence (three injections daily at 2 h intervals; 24 h abstinence) [ 65 ] in mice, nor on RAM performance in rats (2d on, 2d off) [ 75 , 83 ]. However, acute ethanol challenges (1.5 g/kg) after chronic binge-like exposure (2d on, 2d off) resulted in RAM test impairments in both age groups in rats [ 75 , 83 ], with some evidence for increased working memory errors in adolescents [ 83 ].
In sum, there is little evidence for impairments in working memory function in rats after chronic ethanol exposure, with four of the five studies observing no difference between age groups. While acute intoxication impairs working memory function in both ages, there is evidence from only one study that adolescents may make more working memory errors.
Attentional processes: Attentional processing refers to the selection of information that gains access to working memory [ 87 ]. PPI is a pre-attentional cognitive function which provides an index of sensorimotor gating and measures the ability of a lower intensity sensory stimulus to reduce the magnitude of response to a more intense stimulus presented closely afterward. Reduced sensorimotor gating (reduced PPI) can disrupt information processing and thereby impair cognitive function, while enhanced sensorimotor gating (enhanced PPI) may reflect behavioral inflexibility [ 88 ]. For example, lesions in the medial PFC produce both behavioral inflexibility and enhancements in PPI in rats. Two studies assessed attentional processes by measuring prepulse inhibition (PPI) in rats [ 82 , 89 ]. One study observed age-related differences and one did not.
Slawecki and Ehlers [ 82 ] observed age-related differences in sensorimotor gating following ethanol vapor exposure (2w) and brief abstinence (6d), with adolescents showing enhanced PPI at some decibels reflective of behavioral inflexibility, while adults did not exhibit PPI at any of the intensities tested. Slawecki et al. [ 89 ] did not observe any age-related differences in PPI during the acute phase of ethanol withdrawal (7–10 h abstinence) during a period of chronic ethanol exposure (14d).
In sum, there is limited and mixed evidence from two studies of age-related differences in the pre-attentional process of sensorimotor gating. Only one study found support for adolescent sensitivity to ethanol effects.
Cognitive flexibility: Cognitive flexibility refers to the ability to update information based on environmental factors r changing goals in order to adaptively guide decision-making and is linked to the inability to reduce or abstain from drinking [ 90 ]. Three studies examined facets of cognitive and behavioral flexibility [ 79 , 80 , 81 ]. Two of the three studies observed age-related differences.
In two rat studies, cognitive flexibility was assessed using reversal learning paradigms [ 79 , 80 ]. In the reversal learning paradigm, rats were trained on simple (e.g., visual cue) and more complex discriminations (e.g., visual + scent cue) between rewarded and non-rewarded bowls. After learning the discriminants, the rewards were reversed. Ethanol exposure reduced flexibility in both adolescents and adults for simple discriminations in both studies. Age-related differences emerged for the more complex discriminations in one study, with only adults showing reduced flexibility after prolonged abstinence (21d) following binge-like exposure (5 g/kg, 2d on, 2d off) [ 79 ]. In contrast, both age groups showed reduced flexibility for complex discrimination in the other study after prolonged abstinence (21d) despite adolescents consuming more ethanol orally than adults during the 28 week exposure [ 80 ].
In another study, Labots et al. [ 81 ] used a conditioned suppression of alcohol-seeking task after two months of voluntary ethanol consumption (2 months) in rats to examine flexibility around alcohol-seeking behavior. After stratifying the age groups based on levels of ethanol consumption, medium- and high-consuming, adolescents showed higher levels of conditioned suppression compared to similarly drinking adults, indicating greater behavioral flexibility and control over alcohol-seeking in adolescents after chronic voluntary exposure.
Overall, there is limited evidence for adolescent resilience to the effects of chronic alcohol on cognitive flexibility. Two studies found support for adolescent resilience to ethanol’s effect on behavioral flexibility, whereas another study found no differences between adolescents and adults.
Impulsivity: Impulsivity is a multi-faceted behavioral trait that encompasses impaired response inhibition, preference for an immediate reward over a larger but delayed reward, and premature expression of behaviors which may be maladaptive or in conflict with conscious goals. Impulsivity is a risk-factor for the development of addiction and may also be a consequence of sustained substance use [ 35 ]. Pharmacological evidence points towards overlapping neuronal mechanisms in impulsivity and addictive behavior, particularly within the mesolimbic dopamine system [ 91 ]. Two studies examined impulsive decision-making behavior in rats [ 74 , 78 ]. Both studies observed age-related differences.
One study examined impulsive behavior using a delay-discounting task in which choices are made between immediate small rewards and larger delayed rewards [ 78 ]. Regardless of age, chronic intermittent exposure (2d on, 2d off) had no effect on choice behavior in non-intoxicated rats. Following acute challenges, adolescents but not adults demonstrated a reduced preference for the large reward regardless of ethanol exposure history, reflecting a general adolescent-specific heightened impulsivity during intoxication. Another study examined decision-making under risk conditions using an instrumental training and probability-discounting task [ 74 ]. After prolonged abstinence (20d), rats were trained to press two levers for sucrose rewards and were concurrently trained to choose between two levers with different associated probabilities of reward and reward size, creating a choice between a certain, small reward and an uncertain, large reward (i.e., riskier choice). Ethanol consumption was voluntary and while adolescents initially consumed more ethanol than adults at the beginning of the exposure period, the total amount of consumption was similar by the end of the exposure period. Only adolescents showed increased risky and sub-optimal decision-making compared to age-matched controls, while adults performed similarly to controls.
In sum, both studies found support for ethanol having a larger impact on adolescent compared to adults on impulsive behavior.
Goal-directed behavior: Goal-directed behavior refers to when actions are sensitive to both the outcome value (goal) and contingency between the behavior and the outcome [ 92 ]. Two studies used a sign-tracking and omission contingency learning paradigm to examine goal-directed versus habitual behavior [ 76 , 77 ]. One study observed age-related differences and the other did not. Sign tracking refers to tasks where a cue predicts a reward, but no response is needed for the reward to be delivered. Despite this, after repeated pairings of the cue and reward, animals and humans may respond (e.g., via a lever) when the cue is presented anyway, and even when no reward is known to be available. Sign-directed behavior is considered habitual and has been proposed to underlie the lack of control of alcohol use in addiction [ 93 ]. In humans, sign-tracking behavior is difficult to differentiate from goal-directed behavior based on only the observable behavior, i.e., seeing a cue such as a favorite drink or bar and then having a drink [ 94 ]. In the context of alcohol use, reflexively having a drink when seeing an item that is often associated with the rewarding effects of alcohol (e.g., wine glass, bar, smell of alcohol) despite not consciously desiring the alcohol ‘reward’ is an example of how habitual behavior (possibly driven by sign-tracking) can initiate the behavior as opposed to an intentional goal [ 93 ]. Omission contingency refers to a 2nd phase after sign-tracking when the response is punished and the behavior must be inhibited to avoid punishment. After both forced and voluntary ethanol exposure (6w), no alterations to sign-tracking behavior were observed in adolescent and adult rats [ 76 , 77 ]. One study did observe an age-related difference in omission contingency learning, with adolescents performing better than adults after chronic voluntary ethanol exposure [ 77 ]. This preliminarily suggests that adolescents may be more capable of adapting their behavior to avoid punishment compared to adults after chronic use. However, before behavioral testing began, adolescent rats were abstinent for 17 days, while adults were only abstinence for 10 days which may have influenced the results.
In summary, one study found support for adolescents being less sensitive to ethanol effects on goal-directed behavior compared to adults, whereas one study found no effect of ethanol in either age group.
Across the domains of executive function, there is some evidence that adolescents may be more vulnerable to impairments in certain executive and higher-order cognitive functions following chronic alcohol exposure, with increased risky decision-making after prolonged abstinence [ 74 ], impulsivity during intoxication [ 78 ], and reduced working memory function during intoxication after chronic exposure. In contrast, animals exposed to alcohol during adolescence may better retain cognitive flexibility [ 77 , 79 ] and are better able to regain control over alcohol-seeking in adulthood [ 81 ].
Other behavioral outcomes
Anxiety : AUD is highly comorbid with anxiety disorders [ 95 ], especially in adolescence [ 96 ]. While anxiety is not strictly a cognitive outcome, it is related to altered cognitive functioning [ 97 , 98 ]. Many studies assessing the effects of ethanol on the rodent brain and cognition also include anxiety-related measures. Multiple paradigms have been developed to elicit behaviors thought to reflect anxiety in rodents (e.g., rearing, startle, avoidance, etc.). In the open field test (OFT), anxiety is indexed as the tendency to stay close to perimeter walls as animals have a natural aversion to brightly lit open spaces [ 99 ]. In the elevated plus maze paradigm, rodents are placed at the center of an elevated four-arm maze with two open arms two closed arms [ 100 ]. The open arms elicit unconditioned fear of heights/open spaces and the closed arms elicit the proclivity for enclosed, dark spaces. Anxiety is indexed as entries/duration of time in open vs. closed arms, as well as rearing, freezing, or other postural indices of anxiety. In startle paradigms, the startle response is a defensive mechanism reflecting anxiety which follows a sudden, unpredictable stimulus (e.g., tones, light) [ 101 ]. In light-dark box paradigms, anxiety is elicited using a testing apparatus with a light and dark compartment, relying on the conflict between natural aversions to well-lit spaces and the tendency to explore new areas. Percentage of time spent in the light compartment, latency to return to the dark compartment, movement between compartments (transitions), and rearing-behavior are measured as indices of anxiety [ 102 ]. Anxiety can also be assessed using a social interaction test with an unfamiliar partner, with approach and avoidance behaviors measured to index anxiety [ 103 ]. In the novel object test (NOT) [ 104 ], anxiety is elicited by the introduction of a new object in the rodent’s environment. The amount of contacts and time spent in contact with the object is used as an index of anxiety. Similarly, in the marble-burying test (MBT), novel marbles are placed in an environment and the amount of defensive burying of the objects is used as an index of anxiety [ 105 ].
Eleven studies examined anxiety-like behavior in rodents with mixed results across paradigms [ 70 , 78 , 82 , 83 , 89 , 106 , 107 , 108 , 109 , 110 , 111 ]. Overall, five of the eleven studies observed age-related differences.
Two studies used the OFT, finding no effects of voluntary (2w, 4 h/day access) or forced (12/day vapor) ethanol exposure on anxiety-like behavior in adolescents or adult rats during withdrawal (7–9 h) [ 110 ] or after a brief abstinence period (4 days) [ 107 ]. One study used both the MBT and NOT after voluntary ethanol consumption (2 h/d for 2 weeks; no abstinence) and observed higher anxiety in ethanol-exposed adults and reduced anxiety in ethanol-exposed adolescents compared to controls as indexed by marble burying [ 106 ]. However, no age effects were observed in response to a novel object, with reduced interaction with the novel object in both age groups after chronic exposure.
Four studies used the elevated maze paradigm with mixed results. Only one study observed age-related differences in mice after chronic exposure (8–10w vapor) [ 109 ]. Adolescents showed reduced anxiety compared to adults during the acute withdrawal period, but all mice were kept under chronic social isolation and unpredictable stress conditions, which may have affected the results. Two studies in rats found no effect of intermittent (1 g/kg) or binge-like (5 g/kg) exposure in either age group after short (24 h) [ 70 ] or sustained abstinence (20d) [ 83 ]. A third study observed heightened anxiety in both age groups after intermittent exposure (4 g/kg), with anxiety increasing with prolonged abstinence periods (24 h to 12d) [ 108 ].
Three rat studies used a startle paradigm to assess anxiety. Two observed reduced acoustic startle responses after ethanol exposure (12 h/d vapor) in both age groups during acute withdrawal periods (7–10 h) and following more sustained abstinence (6d) [ 82 , 89 ]. In the other study, light-potentiated startle was also reduced in both ages during days 1–10 of withdrawal after binge-like exposure (2d on, 2d off), but age-related differences emerged when the rats were re-exposed via a 4-day binge (1–4/kg). Then, only adults showed higher levels of light-potentiated startle compared to controls [ 78 ], suggesting that ethanol pre-exposure increases anxiety in adults but not adolescents when re-exposed to ethanol after withdrawal.
Two studies used the light-dark box paradigm with mixed results [ 89 , 111 ]. Only adult rats showed increased mild anxiety-like behaviors during early withdrawal (7–10 h) after chronic vapor exposure 12 h/d) [ 89 ]. In contrast, no age-related differences emerged after voluntary ethanol consumption (18 h/d access; 3d/w for 6 weeks), with male mice showing less anxiety-like behavior in both ages [ 111 ]. In contrast, the one study using the social interaction test observed reduced anxiety in adult mice compared to both adolescents and age-matched controls during early withdrawal (4–6 h) after chronic, unpredictable vapor exposure [ 109 ].
In summary, there is inconsistent evidence for age-related differences in the effect of chronic ethanol exposure on anxiety outcomes in rodents. The substantial differences across studies in how anxiety was elicited and measured make it challenging to draw strong conclusions. In the five studies that found age-related differences, adults tend to show higher levels of anxiety, particularly during early withdrawal; however, the opposite was found in the one study examining anxiety in social interactions. Six studies did not observe any age-related differences. Overall, adolescents may be less sensitive to the anxiety-inducing effects of chronic alcohol exposure.
Social behavior: Two studies were identified that examined the effects of chronic ethanol exposure on social behavior in rats [ 112 , 113 ], with both observing age-related differences. After chronic exposure (1 g/kg, 7d), followed by a brief abstinence period (24–48 h), one study found a decrease in social preference in adolescents only [ 112 ], while the other study found no ethanol-related effects on social behavior (2 g/kg, 10d) [ 113 ]. After acute challenges, age and treatment interactions emerged in both studies, but the directions of the results are inconsistent. In the first study, adolescents showed increased social preference, as indexed by the number of cross-overs between compartments toward and away from a peer, across multiple acute doses (0.5–1.0 g/kg) administered immediately before testing, while adults showed no changes in social preference [ 112 ]. In contrast, Morales et al. [ 113 ] found evidence for age-related temporal differences in social activity after acute challenge, with adults showing decreased social impairment five minutes post injection (1 g/kg) and adolescents (1.25 g/kg) after 25 min compared to age-matched controls.
The findings from these two studies paint a complicated and inconsistent picture of the effects of ethanol on social behavior in adults and adolescents warranting further research. One study found support for a larger effect of chronic ethanol on adolescent social behavior compared to adults, while the other did not observe effects of ethanol in either group. One study found support for a larger effect of chronic plus acute ethanol intoxication on social behavior, with the opposite observed in the other.
Brain outcomes
Neurotransmitter systems.
Glutamate is the brain’s main excitatory neurotransmitter and plays a crucial role in synaptic plasticity (i.e., experience-related strengthening or weakening of synaptic connections). Glutamatergic transmission plays an important role in the formation and maintenance of addictive behaviors and the nucleus accumbens (NAc) is considered an important hub in this, receiving glutamatergic input from cortical-limbic areas and dopaminergic input from the midbrain [ 114 ]. Seven studies investigated glutamate functioning in regions of the brain [ 106 , 107 , 108 , 109 , 115 , 116 , 117 , 118 ]. Four of the seven studies observed age-related differences.
Three studies investigated glutamate-related processes in the NAc [ 106 , 107 , 118 ]. Two weeks of voluntary binge drinking (4-h access, no abstinence) did not affect expression of calcium-dependent kinase II alpha (CaMKIIα) and the AMPA receptor GluA1 subunit in the NAc of mice [ 107 ]. In contrast, Lee et al. [ 106 ] showed that voluntary binge drinking (2-h access, no abstinence) increased mGlu1, mGlu5, and GluN2b expression in the shell of the NAc, as well as PKCε and CAMKII in the core of the NAc in adult mice only. In rats, Pascual et al. [ 118 ] showed reduced NR2B phosphorylation in the NAc of adolescents only after two weeks of chronic intermittent ethanol exposure; an effect that also lasted until 24 h after end of exposure. This indicates that adolescents might be less affected by the effects of ethanol on NAc-related glutamatergic neurotransmission than adults. This may in turn mediate decreased withdrawal symptoms and potentially facilitate increased drinking [ 106 ].
Two studies investigated glutamate-related processes in the (basolateral) amygdala [ 107 , 116 ]. In mice, Agoglia et al. [ 107 ] showed decreased CaMKIIα phosphorylation in adolescents, but increased GluA1 expression in adults after two weeks of voluntary binge drinking (4-h access, no abstinence). Also, drug-induced AMPAR activation resulted in increased binge drinking in adolescents but decreased binge drinking in adults, highlighting the potential importance of glutamatergic signaling in age-related differences in alcohol consumption. However, Falco et al. [ 116 ] reported no difference in NR2A mRNA levels in the basolateral amygdala for either age group after 60-day abstinence.
Alcohol’s effects on frontal cortex functioning is thought to be mediated by alterations in NMDA receptor subunit expression [ 119 , 120 ]. Two studies investigated glutamate-related processes in the frontal cortex of rats [ 115 , 118 ]. Pascual et al. [ 118 ] showed reduced NR2B phosphorylation after two weeks of forced intermittent ethanol exposure in adolescents only. Using a 2-week ethanol vapor paradigm, Pian et al. [ 115 ] found different patterns of NMDAR subunit expression. These patterns were highly dependent on abstinence duration (0 h, 24 h, 2w), however, they only statistically compared results within rather than between age groups. Ethanol exposure was associated with decreased NR1 receptor expression in both age groups, but only the adult group showed a decrease in NR2A and NR2B expression. The NR1 and NR2A expression returned to normal during withdrawal, but in adults NR2B expression increased after two weeks of abstinence.
Conrad and Winder [ 109 ] assessed long-term potentiation (LTP) in the bed nucleus stria terminalis (BNST), a major output pathway of the amygdala towards the hypothalamus and thalamus. Voluntary ethanol exposure resulted in blunted LTP responses in the dorsolateral BNST regardless of age. However, all mice were socially isolated during the experiments to induce anxiety, so it is unclear whether the effects were solely due to ethanol exposure.
Two studies looked at glutamate receptor subunit expression in the hippocampus [ 108 , 115 ]. Pian et al. [ 115 ] observed increased expression of NR1, NR2A, and NR2B in adults after 2 weeks of ethanol exposure. In adolescents, a reduction in NR2A expression was observed. After abstinence, adult levels returned to normal, while in adolescents, decreased NR1 and NR2A expression was seen after 24 h but an increased expression of these subunits was seen after 2 weeks of abstinence. These findings support regional specific effects of age group, with potentially increased sensitivity to the impact of alcohol on glutamatergic mediated hippocampal functioning in adolescents. Unlike expected, van Skike et al. [ 108 ] did not find effects of chronic intermittent ethanol exposure or withdrawal on NMDA receptor subunit expression in the hippocampus and cortex as a whole in adolescent and adult rats. The authors speculate that these null results might be associated with the exposure design (limited exposure and route of administration) and lack of withdrawal periods compared to Pian et al. [ 115 ].
In sum, there is limited and inconsistent evidence for age-related differences in glutamate function across seven studies. The direction of the observed age-related differences varies across regions, with evidence of both increased and decreased sensitivity to ethanol effects in adolescents compared to adults in the four studies that observed age-related differences.
GABA is the brain’s main inhibitory neurotransmitter. GABA A receptors are a primary mediator of alcohol’s pharmacological effects [ 121 ]. A total of four studies looked at GABAergic functioning [ 108 , 116 , 122 , 123 ]. Three of the four studies observed age-related differences.
One study investigated GABA-related processes in the (basolateral) amygdala, showing reduced GABA A α1 and GAD67 (enzyme that converts Glutamate to GABA) mRNA expression in adult rats only, 60 days after 18-days ethanol exposure [ 116 ].
Two studies looked at the rat cortex as a whole [ 108 , 122 ]. Van Skike et al. did not find effects of chronic intermittent ethanol exposure on GABA A receptor expression [ 108 ]. Grobin et al. [ 122 ] showed that, while basal GABA A receptor functioning was not affected by 1 month of chronic intermittent ethanol exposure, GABA A receptors were less sensitive to the neurosteroid THDOC in adolescents. This neuromodulatory effect was not found in adults and did not persist after 33 days of abstinence. However, these results indicate that neurosteroids may play an indirect role in age differences in the GABAA receptor’s response to alcohol.
Two studies focused on the rat hippocampus [ 108 , 124 ]. Fleming et al. [ 124 ] found age-specific effects of chronic intermittent ethanol exposure on hippocampal (dentate gyrus) GABA A receptor functioning. Adolescent rats showed decreased tonic inhibitory current amplitudes after ethanol exposure, which was not the case for young adult and adult rats. Also, only the adolescents showed greater sensitivity to (ex vivo) acute ethanol exposure induced enhanced GABAergic tonic currents. The specificity of these effects to adolescent exposure might indicate adolescent vulnerability to ethanol-induced effects on the hippocampus; however, Van Skike et al. [ 108 ] did not find any effects of chronic intermittent ethanol exposure on GABA A receptor expression in the hippocampus.
In sum, given the limited number of studies and lack of replicated effects, no clear conclusions can be drawn about the role of age on the effects of alcohol on GABAergic neurotransmission. Age-specific effects appear to be regionally distinct. The only available study found support for heightened adult sensitivity to ethanol in the amygdala. In contrast, one study found support for greater adolescent sensitivity in the hippocampus and whole cortex, whereas the other found no age-related differences.
The mesocorticolimbic dopamine system, with dopaminergic neurons in the ventral tegmental area (VTA) projecting to the NAc and prefrontal cortex, plays a key role in AUD, particularly through reward and motivational processes [ 14 ]. Only two studies investigated dopaminergic processes, focusing on the frontal cortex, NAc, and broader striatum [ 118 , 125 ]. Both studies observed age-related differences in certain dopamine outcomes.
Carrara-Nascimento et al. [ 125 ] investigated acute effects of ethanol in adolescent and adult mice 5 days after a 15-day treatment with either ethanol or saline. In the PFC, ethanol pretreated adolescents showed reduced dopamine levels (DA) and related metabolites (DOPAC and HVA) in response to an acute ethanol challenge compared to ethanol pretreated adults and adolescent saline controls. In the NAc, there were no differences between pretreated adolescents and adults, but analyses within each age group revealed that ethanol-pretreatment with an acute challenge decreased DOPAC within the adolescent group. Results from the dorsal striatum also showed no differences between adolescents and adults. However, within the adolescent group, ethanol pre-treatment increased DOPAC and, within the adult group, it increased HVA. Pascual et al. [ 118 ] found similar results looking at the expression of DRD1 and DRD2 dopamine receptors after two weeks of chronic intermittent ethanol exposure in rats. In the NAc and dorsal striatum, DRD2 expression was reduced in adolescent compared to adult exposed rats, while both DRD1 and DRD2 expression were reduced in the frontal cortex.
These results suggest reduced alcohol-induced dopamine reactivity in adolescents in the PFC and NAc based on the two available studies, but more studies are warranted for a more detailed understanding of the relationship between age and dopamine receptor expression following chronic ethanol exposure.
Acetylcholine
Acetylcholine is a known neuromodulator of reward and cognition-related processes [ 126 ]. The composition and expression of nicotinic and muscarinic acetylcholine receptors have been implicated in various alcohol use-related behaviors [ 127 , 128 ]. Only one study investigated cholinergic processes and observed age-related differences. Vetreno et al. [ 129 ] showed global reductions in choline acetyltransferase (ChAT; cholinergic cell marker) expression after adolescent onset, but not adult onset of forced intermittent binge-like exposure (20 days – every other day, 25 days abstinence).
Neuromodulatory processes
Neurodegeneration and neurodevelopment.
Chronic alcohol consumption is thought to lead to brain damage by influencing processes involved in neurodegeneration and neurogenesis. The formation of addictive behaviors is paralleled by the formation of new axons and dendrites, strengthening specific neuronal pathways [ 130 ]. While brain morphology is commonly investigated in humans, it is a proxy of the impact of alcohol on the brain and therefore rarely studied in rodents. Five studies investigated facets of neurodegeneration or development in rodents [ 55 , 65 , 131 , 132 , 133 ]. All five studies observed age-related differences.
Huang et al. [ 131 ] showed reduced cerebral cortex mass in adolescent mice, but shortening of the corpus collosum in adults after 45 days of ethanol injections, suggesting some age-specific regional effects. Using an amino cupric silver staining, significant brain damage was revealed for both adolescent and adult rats after 4 days of binge-like ethanol exposure [ 132 ]. However, adolescents showed more damage in the olfactory-frontal cortex, perirhinal cortex, and piriform cortex.
Looking at hippocampal neurogenesis, ethanol exposure has been shown to initially reduce hippocampal neurogenesis in adult rodents, recovering after 1-month abstinence [ 134 ]. Compared to adults, neurogenesis in the dentate gyrus of the hippocampus was found to be reduced in adolescent exposed mice (Bromodeoxyuridine levels) [ 65 ] and rats (doublecortin levels) [ 133 ]. Lacaille et al. [ 65 ] also measured the expression level of genes involved in oxidative mechanisms after binge-like alcohol exposure. In whole brain samples, they found increased expression of genes involved in brain protection (i.e., gpx3, srxn1) in adults, but increased expression of genes involved in cell death (i.e., casp3) combined with decreased expression of genes involved in brain protection (i.e., gpx7, nudt15) in adolescents. Casp3 protein levels were also higher in the whole brain of adolescent exposed mice [ 65 ] and the adolescent dentate gyrus [ 133 ], suggesting more neurodegeneration and less neurogenesis in adolescents versus adults following ethanol consumption.
Cyclin-dependent kinase 5 (CDK5) is involved in axon, dendrite, and synapse formation and regulation. CDK5 is overexpressed in the prefrontal cortex and the NAc following exposure to substances of abuse including alcohol [ 135 ]. Moreover, CDK5 inhibition has been shown to reduce operant self-administration of alcohol in alcohol-dependent rats [ 136 ]. One study reported higher H4 acetylation of the CDK5 promoter in the PFC of adult versus adolescent ethanol-exposed rats during acute withdrawal, however, CDK5 mRNA expression was control-like after 2 weeks of abstinence [ 55 ].
In sum, strong conclusions cannot be drawn due to the limited number of studies and lack of replicated effects. However, preliminary evidence points to adolescent vulnerability to damage in the cortex, reduced neurogenesis, and increased neurodegeneration in the hippocampus and the cortex as a whole based on four of the five studies. In contrast, one study found support for adult vulnerability to ethanol’s effects axon, dendrite, and synapse formation and regulation.
Growth factors
Brain-derived neurotrophic factor (BNDF) and nerve growth factor (NGF) are involved in brain homeostasis and neural recovery [ 137 , 138 ]. While ethanol exposure initially increases BDNF and NGF, chronic ethanol exposure seems to reduce BDNF and NGF levels and can thereby result in long-term brain damage and related cognitive problems [ 139 , 140 ]. Four studies investigated growth factor expression in the frontal cortex [ 54 , 55 , 79 , 80 ] and two studies also investigated the hippocampus [ 79 , 80 ]. All four studies of the frontal cortex observed age-related differences. Neither study of the hippocampus observed age-related differences.
In rats, 30 weeks of chronic ethanol exposure reduced prefrontal mBDNF and β-NGF regardless of age, despite adolescents consuming more ethanol [ 80 ]. Moreover, the reduction of mBDNF was correlated with higher blood alcohol levels and was persistent up to 6–8 weeks abstinence. Interestingly, during acute withdrawal (48 h) adolescents but not adults temporarily showed control-like mBDNF levels. This might indicate an attempt to counteract neurodegeneration as a result of ethanol exposure in adolescents. These results were partially replicated using a shorter intermittent exposure paradigm (13 doses, 2 days on/off) [ 79 ]. While intoxication after chronic ethanol exposure reduced prefrontal BDNF, levels recovered after 3-weeks abstinence regardless of age. However, during acute withdrawal (24 h), BDNF was still reduced in early-adolescent onset rats, increased in adult-onset rats, but control-like in mid-adolescent onset-rats, suggesting slower recovery in younger animals. Looking at BDNF gene regulation, a similar study (8 doses, 2 days on/off) reported higher H3 demethylation but lower H4 acetylation of the BDNF promoter in the PFC of adult versus adolescent ethanol-exposed rats during acute withdrawal [ 55 ]. However, prefrontal BDNF mRNA expression returned to control levels after 2 weeks of abstinence. Interestingly, social housing may be protective, as reduced prefrontal BDNF was no longer observed in alcohol-exposed adolescent mice housed in environmentally enriched relative to standard conditions [ 54 ]. Two studies investigated hippocampal BDNF expression but reported no significant interactions between alcohol exposure and age group [ 79 , 80 ].
In sum, the results of the four available studies suggest lower prefrontal BDNF during chronic alcohol use that recovers after abstinence regardless of age. However, the rate of recovery may be influenced by age with slower recovery in adolescents. In the two available studies, no age-related differences were observed in BDNF expression in the hippocampus.
Transcription factors
The transcription factors cFos and FosB are transiently upregulated in response to substance use, and ΔFosB accumulates after chronic exposure, particularly in striatal and other reward-related areas [ 141 ]. Two studies investigated cFos and FosB [ 55 , 142 ] and one study ΔFosB related processes [ 111 ]. All three studies observed age-related differences.
After chronic ethanol exposure (8 doses, 2 days on/off), adolescent compared to adult rats showed increased prefrontal H3 and H4 acetylation of the cFos promotor region and increased H4 acetylation and H3 dimethylation of FosB promotor regions after acute abstinence [ 55 ]. Moreover, mRNA expression of FosB was elevated in adolescents but not adults after 2-weeks abstinence. The upregulating effects of an acute ethanol challenge on prefrontal cFos appears to reduce after chronic pre-treatment to a larger extent in adolescent than adult exposed mice [ 142 ]. This pattern of results was similar in the NAc, but desensitization to ethanol’s acute effects on cFos in the hippocampus was more pronounced in adults. Faria et al. [ 142 ] also looked at Egr-1 (transcription factor, indirect marker of neuronal activity and involved in neuroplasticity), showing a stronger reduction in Egr-1 expression in the PFC, NAc, and hippocampus of adolescent versus adults after repeated ethanol exposure. Regarding ∆FosB, Wille-Bille et al. [ 111 ] found increased ∆FosB in adolescent compared to adult rats in the prelimbic PFC, dorsomedial striatum, NAc core and shell, central amygdala nucleus capsular, and basolateral amygdala after 3 days per week 18 h ethanol exposure sessions for 6 weeks. In sum, the three available studies provide preliminary evidence for increased adolescent vulnerability to ethanol-induced long-term genetic (mRNA expression) and epigenetic (methylation) changes in mesocorticolimbic areas.
Immune factors
Ethanol is known to trigger immune responses in the brain (e.g., increase production of hemokines and cytokines), causing inflammation and oxidative stress [ 143 , 144 , 145 ]. Three studies examined immune factors [ 146 , 147 , 148 ]. Two of the three studies observed age-related differences.
Microglia remove damaged brain tissue and infectious agents and are key to the brain’s immune defense. Only one study investigated microglia levels [ 146 ]. Although direct comparisons between age groups were missing, both adolescent and adult rats showed less microglia in the hippocampus (CA and DG) and peri-entorhinal cortex, and more dysmorphic microglia in the hippocampus after 2 and 4 days of binge-like ethanol exposure [ 146 ]. Notably, age groups were matched on intoxication scores, with adolescents needing more ethanol to reach the same level of intoxication. An in silico transcriptome analysis of brain samples from mice after 4 days of 4 h/day drinking in the dark, suggest overexpression of neuroimmune pathways related to microglia action (toll-like receptor signaling, MAPK signaling, Jak-STAT signaling, T-cell signaling, and chemokine signaling) in adults that was not observed in adolescents, while adolescents consumed more ethanol [ 147 ]. Similarly, ethanol-exposed adult mice showed higher chemokine expression (CCL2/MCP-1) in the hippocampus, cerebral cortex, and cerebellum and higher cytokine expression (IL-6, but not TNF-α) in the cerebellum, while no chemokine or cytokine changes were observed in ethanol exposed adolescent mice [ 148 ]. Both adolescents and adults showed increased astrocyte levels in the hippocampus (CA1) and the cerebellum after ethanol exposure, but changes in astrocyte morphology were only observed in the adult hippocampus.
In sum, two of the studies found support for increased immune responses after ethanol exposure in adults compared to adolescents, whereas the one other study found no difference between the age groups.
HPA-axis functionality
Chronic stress and HPA-axis functionality have been associated with the maintenance of AUD (e.g., reinstatement drug seeking, withdrawal) [ 149 ]. Two studies investigated corticotropin-release factor (CRF) expression in rats [ 116 , 150 ]. One study observed age-related differences and the other did not.
Falco et al. [ 116 ] found decreased CRF mRNA expression in the adult but not adolescent basolateral amygdala 2 months after 18-day restricted ethanol exposure. In contrast, Slawecki et al. did not find any interaction between age and treatment on CRF levels in the amygdala, as well as the frontal lobe, hippocampus, hypothalamus, and caudate 7 weeks after 10-days of ethanol vapor exposure.
No conclusions can be drawn. One study observed found support for reduced effects of ethanol on HPA-axis functionality compared to adults, whereas the other observed no difference between the age groups. Future studies using different (voluntary) exposure paradigms are needed to further investigate the effects of alcohol on HPA activity in relation to age of alcohol exposure.
Neuropeptides
Neuropeptides are a diverse class of proteins that have a modulatory function in many different processes, including but not limited to neurotransmission, stress, immune responses, homeostasis, and pain [ 151 , 152 , 153 ]. Only one study investigated neuropeptides in rats and observed age-related differences [ 150 ].
Slawecki et al. [ 150 ] specifically investigated neuropeptide-Y, substance-P, and interleukine expression in the frontal lobe, hippocampus, hypothalamus, dorsal striatum, and amygdala 7 weeks after 10-days of ethanol vapor exposure in rats [ 150 ]. Interactions between age and treatment were found for the hippocampus and caudate only. Ethanol-induced reductions in hippocampal neuropeptide-Y and increases in caudate neurokinine were more pronounced in adults compared to adolescents suggesting long-lasting effects of ethanol in adults but not adolescents.
Ethanol metabolism
The first metabolite of ethanol is acetaldehyde, which has been theorized to mediate the effects of ethanol on both brain and behavior [ 154 ]. Only one study investigated ethanol metabolism in the brain and did not observe age-related differences [ 155 ].
Rhoads et al. showed that despite the fact that adolescent rats consumed more alcohol brain catalase levels after 3-weeks of ethanol exposure (no abstinence) did not differ between adolescents and adults [ 155 ]. Although the general role of catalase in ethanol metabolism is small, catalase can oxidize ethanol to acetaldehyde in the brain, affecting elimination of ethanol after consumption [ 156 , 157 ]. These findings may therefore imply that ethanol metabolism may not differ between adolescent and adult animals, which should be studied in a more direct manner.
Full proteome analysis
While the previously described studies focused on specific factors involved in neurotransmission, brain health, and plasticity, proteomics allows for the study of the full proteome in a specific region or tissue type. One study investigated the impact of age on ethanol-induced changes in the hippocampal proteome, observing age-related differences [ 158 ]. In this study, rats intermittently and voluntarily consumed beer for 1 month and the hippocampal proteome was analyzed after 2 weeks of abstinence. The results point to the involvement of many of the factors described above and imply age-specific effects of alcohol. Adult beer exposure increased citrate synthase (part of the citric acid, or Krebs, cycle) and fatty acid binding proteins (involved in membrane transport) compared to controls. Adolescent beer exposure increased cytoskeletal protein T-complex protein 1 subunit epsilon (TCP-1), involved in ATP-dependent protein folding, and reduced expression of a variety of other proteins involved in glycolysis, glutamate expression, aldehyde detoxification, protein degradation, and synaptogenesis, as well as neurotransmitter release. These more extensive changes suggest that the adolescent hippocampus might be more vulnerable to the effects of ethanol exposure, but more studies are needed to clarify and replicate these findings and extend the focus to different brain areas.
Neuronal activity and functioning
Ethanol-induced molecular changes may eventually change neuronal activity. Three studies investigated neuronal activity and functioning [ 89 , 159 , 160 ] using electrophysiological methods. All three studies observed age-related differences.
Galaj et al. [ 159 ] assessed firing patterns and the structure of pyramidal neurons in the L2 and L5 layers of the prelimbic cortex of the rat brain using ex vivo electrophysiological recordings and morphological staining. Following chronic intermittent ethanol exposure and brief abstinence (2 days), adolescents, but not adults, showed reduced amplitudes of spontaneous excitatory post-synaptic currents (sEPSCs) in L5 neurons compared to controls, indicating reductions in intrinsic excitability. In line with this, Dil staining showed increased thin spine ratios in the L5 layer in adolescents only. Age differences were more pronounced after prolonged abstinence (21 days), with adolescents showing reduced amplitude and frequency of sEPSCs in L5 neurons while adult’s L5 neurons showed augmented firing patterns (i.e., amplitude and frequency). Furthermore, adolescent rats showed decreased total spine density and non-thin spines, indicating less excitatory postsynaptic receptors in the L5 layer. In contrast, adults showed increases in spine density and non-thin spines.
Li et al. [ 160 ] examined the functioning of CA1 interneurons, which are important for learning and memory processes [ 161 ], in the rat hippocampus using ex vivo whole-cell recordings. After prolonged abstinence (20 days), voltage-gated A-type potassium channel ( I A ) conductance was measured. Differences emerged between age groups (although no statistical interaction effect was directly assessed): EtOH-exposed adolescents and adults both showed lower I A mean peak amplitude compared to the respective control groups. However, adolescents also showed reduced I A density and increased mean decay time, which decreased in adults. Furthermore, only adolescents showed increased depolarization required for activation compared to controls, which can result in higher interneuron firing rates in the CA1 region that could affect learning processes. Additional research is needed to connect these findings to behavioral measures of learning and memory.
Slawecki et al. [ 89 ] was the only study to use in vivo electroencephalogram (EEG) recordings with rats to examine function in the frontal and parietal cortex at different times during a 14-day vapor exposure period. During acute withdrawal (7–10 h abstinence period), following daily exposure no effects emerged in frontal cortical regions throughout the exposure period. In parietal regions, only adolescents showed increased high frequency (16–32 Hz and 32–50 Hz) power on days 8 and 12 compared to controls. Adolescent hyperexcitability during withdrawal may indicate increased arousal in adolescents compared to adults during withdrawal, but more studies linking brain activity to behavioral indices of withdrawal will allow for clearer interpretations.
Overall, strong conclusions cannot be drawn given the disparate paradigms and outcomes utilized. While adolescents and adults appear to differ in the effect of ethanol on neuronal firing, the meaning of these differences is not clear given the lack of connection between these findings and behavioral outcomes.
Human studies
Four studies examined age-related differences of the effect of alcohol on brain or cognition in humans [ 162 , 163 , 164 , 165 ].
Müller-Oehring et al. [ 162 ] examined the moderating role of age on resting state functional connectivity and synchrony in the default mode, central executive, salience, emotion, and reward networks of the brain in a sample of no/low and heavier drinkers aged 12–21 years old. While the study did not compare discrete groups of adolescents and adults, analyses investigating the interaction between continuous age and alcohol exposure history were conducted which provide insight into the effect of alcohol use on functional brain networks from early adolescence to emerging adulthood. Regardless of age, no differences were observed between matched subgroups of no/low drinkers and moderate/heavy drinkers in the default mode, salience, or reward networks. However, in the central executive network, connectivity between the superior frontal gyrus (SFG) and insula increased with age in the no/low drinkers but not in heavier drinkers. Age-related strengthening of this fronto-limbic connection correlated with better performance on a delay discounting task in boys, suggesting that adolescent alcohol use may interfere with typical development of higher-level cognitive functions. In the emotion network, amygdala-medial parietal functional synchrony was reduced in the heavier drinkers compared to the no/low drinkers and exploratory analyses suggested that weaker amygdala-precuneus/posterior cingulate connectivity related to later stages of pubertal development in the no/low drinking group only. Interestingly, in the default mode (posterior cingulate-right hippocampus/amygdala) and emotional networks (amygdala, cerebellum), connectivity in regions that exhibited age-related desynchronization was negatively correlated with episodic memory performance in the heavy drinkers. These results give preliminary evidence that alcohol might have age-dependent effects on resting state connectivity and synchronization in the central executive, emotion, and default mode networks that could potentially interfere with normative maturation of these networks during adolescence.
Three studies examined age effects in alcohol-related implicit cognitions, specifically attentional bias [ 163 , 165 ], alcohol approach bias [ 165 ], and implicit memory associations and explicit outcome expectancies [ 164 ]. Attentional bias refers to the preferential automatic allocation or maintenance of attention to alcohol-related cues compared to neutral cues which is correlated with alcohol use severity and craving [ 166 ]. McAteer et al. [ 163 ] measured attentional bias with eye tracking during presentation of alcohol and neutral stimuli in heavy and light drinkers in early adolescents (12–13 yrs), late adolescents (16–17 yrs), and young adults (18–21 yrs). Regardless of age, heavy drinkers spent longer fixating on alcohol cues compared to light drinkers. Cousijn et al. [ 165 ] measured attentional bias with an Alcohol Stroop task [ 167 ], comparing the speed of naming the print color of alcohol-related and control words. Consistent with the findings of McAteer et al. [ 163 ], adults and adolescents matched on monthly alcohol consumption showed similar levels of alcohol attentional bias. In the same study, Cousijn et al. [ 165 ] did not find any evidence for an approach bias towards alcohol cues in any age group.
Rooke and Hine [ 164 ] found evidence for age-related differences in implicit and explicit alcohol cognitions and their relationship with binge drinking. Using a teen-parent dyad design, adolescents (13–19 yrs) showed stronger memory associations in an associative phrase completion task and more positive explicit alcohol expectancies than adults. Interestingly, both explicit positive alcohol expectancies and implicit memory associations were a stronger predictor of binge drinking in adolescents compared to adults. It is important to note that adolescents also had higher levels of binge drinking than adults in the study.
Cousijn et al. [ 165 ] also investigated impulsivity, drinking motives, risky decision-making, interference control, and working memory. No age differences emerged in the cognitive functioning measures including risky decision-making (Columbia Card Task – “hot” version), interference control (Classical Stroop Task), or working memory (Self-Ordered Pointing Task). However, adolescents were more impulsive (Barrett Impulsiveness Scale) than adults and reported more enhancement motives. Importantly, impulsivity as well as social, coping, and enhancement motives of alcohol use correlated with alcohol use in both ages. However, age only moderated the relationship between social drinking motives and alcohol use-related problems (as measured by the Alcohol Use Disorder Identification Test), with a stronger positive association in adolescents compared to adults. Importantly, the adolescent group had a different pattern of drinking, with less drinking days per month but more drinks per episode than the adult group.
In summary, human evidence is largely missing, with no studies comparing more severe and dependent levels of alcohol use between adolescents and adults. The preliminary evidence is too weak and heterogeneous to draw conclusions, warranting future studies investigating the impact of age.
The current systematic review assessed the evidence for the moderating role of age in the effects of chronic alcohol use on the brain and cognition. The identified 59 rodent studies (Table 1 ) and 4 human studies (Table 2 ) provide initial evidence for the presence of age-related differences. Rodents exposed to ethanol during adolescence show both increased risk and resilience to the effects of ethanol depending on the outcome parameter. However, due to the high variability in the outcomes studied and the limited number of studies per outcome, conclusions should be considered preliminary. Moreover, brain and behavioral outcomes were mostly studied separately, with studies focusing on either brain or behavioral outcomes. The behavioral consequences of changes in certain brain outcomes still need to be investigated. Table 3 provides a comprehensive overview of the strength of the evidence for age-related differences for all outcomes. Below, we will discuss the most consistent patterns of results, make connections between the behavioral and neurobiological findings when possible, highlight strengths and limitations of the evidence base, and identify the most prominent research gaps.
Patterns of results
Age-related differences in learning and memory-related processes appear to be highly domain specific. There is limited but fairly consistent evidence for adolescent-specific impairments in contextual fear conditioning, which could be related to hippocampal dysfunction. Results for other hippocampus-related memory processes such as spatial memory are mixed and largely based on forced exposure with acute challenge studies rather than voluntary long-term exposure to alcohol. The evidence base is currently insufficient to draw conclusions about the role of age in alcohol’s effects on non-spatial types of learning and memory. Alcohol generally did not impact performance in the non-spatial variants of the MWM and SBM paradigms or in reward-learning, but the results of the limited studies in the object-learning domain highlight potential impairments and the importance of age therein. For example, adolescents but not adults demonstrated impaired object memory in the only study using the novel object recognition task [ 65 ]. Acute challenges after chronic pre-exposure to alcohol also appear to impair performance in the working memory domain, with one study suggesting heightened adolescent sensitivity to working memory impairment [ 83 ]. Thus, although the domain-specific evidence is limited by the relative lack of research, overall patterns suggest that learning and memory functions that are primarily hippocampus-dependent may be differentially affected by adolescent compared to adult alcohol use. Studies focusing on neural hippocampal processes corroborate these findings, reporting more extensive changes in protein expression [ 158 ], less desensitization of cFos upregulation [ 142 ], larger changes in GABAa receptor subunit expression [ 124 ], longer lasting changes in NMDA receptor expression [ 115 ], and larger reductions in neurogenesis [ 65 , 133 ] in the hippocampus of adolescent compared to adult ethanol-exposed rodents. On the other hand, ethanol-induced changes in the hippocampus recovered more quickly in younger animals after abstinence [ 150 ] and adolescent mice showed less signs of ethanol-induced neuroinflammation compared to adults [ 148 ].
Higher rates of adolescent alcohol use, especially binge drinking, may be facilitated by a heightened sensitivity to the rewarding properties of alcohol in combination with a reduced sensitivity to the negative effects of high doses [ 47 ]. In line with this, there is limited but consistent evidence that adolescents show less CTA in response to chronic ethanol and consequently voluntarily consume more ethanol [ 50 ]. Importantly, distinct vulnerability periods within adolescence for altered CTA may exist [ 168 , 169 ], with early adolescents potentially being least sensitive to aversive effects. Future studies using chronic exposure paradigms comparing different stages of adolescence to adults are needed. In contrast to CTA, there is insufficient evidence of age-related differences in the motivational value of alcohol based on CPP paradigms, with only one of five studies reporting stronger CPP in adolescents than adults [ 52 ]. Adolescents may be more sensitive to the effects of environmental factors on the motivational value of alcohol than adults, as adolescents housed in enriched environments acquired CPP while those in standard housing did not, an effect that was not found in adults [ 54 ]. Evidence for environmentally enriched housing being protective against these changes in adolescents provides an important indication that environmental factors matter and are important factors to consider in future research on the motivational value of ethanol on both the behavioral and neural level. Complementary studies on the functioning of brain regions within the mesolimbic dopamine pathway and PFC, which play an important role in motivated behavior, indicate limited but consistent evidence for age-related differences. Adolescents showed less dopamine reactivity in the PFC and NAc compared to adults after chronic ethanol exposure. Furthermore, there is limited but consistent evidence that adolescents are more vulnerable to epigenetic changes in the frontal cortex and reward-related areas after chronic ethanol exposure. For instance, adolescents may be more sensitive to histone acetylation of transcription factors in motivational circuits underlying the rewarding effects of alcohol [ 55 ], which may contribute to addictive behaviors [ 170 , 171 ]. Chronic alcohol use is also associated with lower BDNF levels in the PFC and subsequent increases in alcohol consumption, implicating BDNF as an important regulator of alcohol intake [ 172 ]. While evidence is limited, chronic alcohol use consistently reduced prefrontal BDNF in both age groups. However, the rate of recovery of BDNF levels after abstinence appears to be slower in adolescents.
Regarding executive functioning, there is limited but fairly consistent evidence from animal studies that adolescents are more vulnerable to long-term effects of chronic exposure on decision-making and are more impulsive than adults during acute intoxication and after prolonged abstinence following chronic exposure. Impulsivity is associated with functional alterations of the limbic cortico-striatal systems [ 91 ], with involvement of both the dopaminergic and serotonergic neurotransmitter systems [ 173 ]. While no studies investigating serotonergic activity were identified, the consistent reduction in dopamine reactivity observed in the PFC and NAc in adolescents compared to adults parallel the behavioral findings. There is also limited but fairly consistent evidence that adolescents are more resilient to impairments in cognitive flexibility than adults following chronic exposure to alcohol, and that adolescents may more easily regain control over their alcohol-seeking behavior than adults. These behavioral findings provide preliminary support for the paradox of adolescent risk and resilience in which adolescents are at once more at risk to develop harmful patterns of drinking, but are also more resilient in that they may be more equipped to flexibly change behavior and with time regain control over alcohol consumption. However, studies assessing processes that might be related to brain recovery provide little conclusive evidence for potential underlying mechanisms of these behavioral findings. While adolescents appear more vulnerable to ethanol-induced brain damage [ 131 , 132 ], show reduced neurogenesis [ 65 , 133 ], and show less changes in gene expression associated with brain recovery [ 65 , 133 ], adults show relatively higher immune responses after repeated ethanol exposure [ 147 , 148 ]. The limited evidence for adolescent resilience to alcohol’s effects on cognitive flexibility diverge from the conclusions of recent reviews that focused mostly on adolescent-specific research. Spear et al. [ 18 ] concluded that adolescents are more sensitive to impairments in cognitive flexibility; however, this was based on adolescent-only animal studies. Similarly, the systematic review of Carbia et al. [ 19 ] on the neuropsychological effects of binge drinking in adolescents and young adults also revealed impairments in executive functions, particularly inhibitory control. However, as pointed out by the authors, the lack of consideration of confounding variables (e.g., other drug use, psychiatric comorbidities, etc.) in the individual studies and the lack of prospective longitudinal studies limit our ability to causally interpret these results. This further highlights the difficulty of conducting human studies which elucidate causal associations of the effects of alcohol, and the need for animal research that directly compares adolescents to adults to bolster interpretation of findings from human research.
Only a few studies have investigated age-related differences in cognitive functioning in humans. These studies focused on mostly non-dependent users and studied different outcomes, including cognitive biases and implicit and explicit alcohol-related cognitions. Overall, there was limited but consistent evidence that age does not affect alcohol attentional or approach biases, with heavy drinkers in both age groups allocating more attention to alcohol cues compared to controls [ 163 , 165 ]. In contrast, in line with a recent meta-analysis of the neurocognitive profile of binge-drinkers aged 10–24 [ 23 ], there is limited evidence that age affects alcohol associations. One study found age effects on implicit (memory associations) and explicit (expectancies) cognition in relation to alcohol use. Adolescents showed stronger memory associations and more positive expectancies than adults [ 164 ]. These expectancies were also predictive of higher binge drinking in adolescents but not adults, highlighting the importance of future research into age differences in alcohol-related cognitions and their consequences on alcohol consumption. However, the quality of the evidence was rated as weak based on the methodological design of the included studies.
Regarding anxiety-related outcomes, results are inconsistent across studies and paradigms. When age-differences are observed, adolescents often show reduced anxiety compared to adults during both acute withdrawal and sustained abstinence following chronic ethanol exposure. However, the direction of age-related effects of alcohol may also be anxiety-domain specific. In social settings, adults show reduced anxiety compared to adolescents. Research on the neurocircuitry of anxiety processes implicates the extended amygdala, especially the BNST, in anxiety behaviors with an emphasis on the role of GABAergic projections to the limbic, hindbrain, and cortical structures in rodents [ 174 ]. Despite adolescents showing less non-social anxiety than adults after ethanol exposure, no age-differences were observed for LTP in the BNST [ 109 ]. Also, GABA receptor expression in the hippocampus and whole cortex was not altered by ethanol exposure in either age group [ 108 ]. However, the anxiolytic effects of NMDA antagonists [ 175 ] also highlight the importance of glutamatergic activity in anxiety processes [ 176 ]. In line with behavioral findings, adolescents were less sensitive to changes in glutamate expression: adults showed heightened expression in the NAc, which has been suggested to underlie the higher levels of anxiety observed in adults compared to adolescents [ 106 ]. Importantly, across the various studies, different paradigms were used to assess anxiety, potentially contributing to the inconsistent results. Furthermore, most of the identified studies used a forced ethanol exposure paradigm. As alcohol-induced anxiety is likely also dependent on individual trait anxiety, voluntary consumption studies in high and low trait anxiety animals are important to further our understanding of the interaction between alcohol use and anxiety. Of note, the observed pattern suggestive of reduced anxiety in adolescents compared to adults diverges from conclusions of previous reviews such as Spear et al. [ 18 ] which concluded that adolescents are more likely to show augmented anxiety after alcohol exposure based on animal studies with adolescent animals only. Importantly, anxiety was included as a secondary outcome in this review because of the high comorbidity between anxiety disorders and alcohol addiction, warranting the inclusion of age-related differences in the relation between alcohol and anxiety. However, the search strategy was not specifically tailored to capturing all studies assessing age-related differences in the effect of alcohol on anxiety.
Translational considerations, limitations, and future directions
The reviewed studies revealed a high degree of variability in study designs and outcomes, hindering integration and evaluation of research findings. We were unable to differentiate our conclusions based on drinking patterns (i.e., comparing binge drinking, heavy prolonged use, AUD). The prevalence of binge-drinking in adolescence is very high and is associated with neurocognitive alterations [ 177 ]. Studies investigating the potential differential impact of binge-drinking compared to non-binge-like heavy alcohol use in adolescence and adulthood are critical for understanding the risks of chronic binge-like exposure in adolescence, even if it does not progress to AUD.
It is also important to acknowledge the limitations of the choice of adolescent and adult age ranges in our inclusion criteria. Rodent studies had to include an adolescent group exposed to alcohol between the ages of PND 25–42 and an adult group exposed after age PND 65. Ontogenetic changes may still be occurring between PND 42–55, and this period may more closely correspond to late adolescence and emerging adulthood in humans (e.g., 18–25 years). Studies that compared animals in this post-pubertal but pre-adulthood age range were not reviewed. Studies investigating age-related differences in the effects of ethanol on brain and cognitive outcomes in emerging adulthood are also translationally valuable given the high rates and risky patterns of drinking observed during this developmental period [ 178 ]. Indeed, an important future direction is to examine whether there are distinct vulnerability periods within adolescence itself for the effects of ethanol on brain and cognitive outcomes. Given that emerging adulthood is a period of continued neurocognitive maturation and heightened neural plasticity, studies comparing this age range to older adults (e.g., over 30) are also necessary for a more thorough understanding of periods of risk and resilience to the effects of alcohol.
Furthermore, we did not conduct a risk of bias assessment to examine the methodological quality of the animal studies. The applicability and validity of the risk of bias tools for general animal intervention studies, such as the SYRCLE risk of bias tool [ 179 ], remain in question at the moment. The lack of standardized reporting in the literature for many of the criteria (e.g., process of randomizing animals into intervention groups) would lead to many studies being labeled with an ‘unclear risk of bias’. Furthermore, there is still a lack of empirical evidence regarding the impact of the criteria in these tools on bias [ 179 , 180 ]. This is a significant limitation in evaluating the strength of the evidence for age-related differences based on the animal studies, which highlights the importance of more rigorous reporting standards in animal studies.
Moreover, most work is done in male rodents and is based on forced ethanol exposure regimes. In a recent opinion article, Field and Kersbergen [ 181 ] question the usefulness of these types of animal models to further our understanding of human substance use disorders (SUD). They argue that animal research has failed to deliver effective SUD treatment and that social, cultural, and other environmental factors crucial to human SUD are difficult, if not impossible, to model in animals. While it is clear that more sophisticated multi-symptom models incorporating social factors are needed to further our understanding of SUD and AUD specifically, a translational approach is still crucial in the context of investigating the more fundamental impact of alcohol use on brain and cognition. In humans, comparing the impact of alcohol use on brain and cognition between adolescents and adults is complicated by associations between age and cumulative exposure to alcohol; i.e., the older the individual, the longer and higher the overall exposure to alcohol. Although animal models may be limited in their ability to model every symptom of AUD, they can still provide critical insights into causal mechanisms underlying AUD by allowing direct control over alcohol exposure and in-depth investigation of brain mechanisms.
The intermittent voluntary access protocol resembles the patterns of alcohol use observed in humans, and also result in physiologically relevant levels of alcohol intake [ 182 , 183 , 184 ]. Only a minority of the studies included in this review employed a voluntary access protocol, with one study using beer instead of ethanol in water [ 158 ], which better accounts for the involvement of additional factors (e.g., sugar, taste) in the appeal of human alcohol consumption. Voluntary access protocols can also model behavioral aspects of addictive behavior such as loss of control over substance use and relapse [ 185 , 186 , 187 ], an important area in which little is known about the role of age. Ideally, one would also investigate choices between ethanol and alternative reinforcers, such as food or social interaction, that better mimic human decision-making processes [ 188 ]. However, studies on the effects of ethanol on social behavior are limited and show inconsistent results and studies assessing reward processes often lack a social reward component as an alternative reinforcer.
On a practical level, rodents mature quickly and choice-based exposure paradigms are more complex and time-consuming than most forced exposure paradigms. Consequently, by the time final behavioral measurements are recorded, both the adolescent and adult exposure groups have reached adulthood. To combat this, many of the included studies use forced ethanol exposure, such as ethanol vapor, to quickly expose rodents to very high doses of ethanol. Although the means and degrees of alcohol exposure may not directly translate to human patterns of alcohol use, such studies do allow for the assessment of the impact of high cumulative doses of ethanol within a relatively short period of time which allows for more time in the developmental window to test age-related differences in the outcomes. When considering the translational value of a study, it is therefore important to evaluate studies based on the goal, while not ignoring the practical constraints.
While human research is challenging due to the lack of experimental control and the inherent confounds in observational studies between age and alcohol exposure history, large-scale prospective longitudinal studies offer a gateway towards a better understanding. Comparisons of different trajectories of drinking from adolescence to adulthood (i.e., heavy drinking to light drinking, light drinking to heavy drinking, continuously heavy drinking, and continuously light drinking) could offer insight into the associated effects on cognitive and brain-related outcomes. Of course, different drinking trajectories are likely confounded with potentially relevant covariates which limits causal inference. Direct comparisons of low and heavy adolescent and adult drinkers, supported by a parallel animal model can help to bolster the causality of observed age-related differences in human studies. In addition, changes in legislation around the minimum age for alcohol consumption in some countries provide a unique opportunity to investigate how delaying alcohol use to later in adolescence or even young adulthood impacts cognitive functioning over time. Importantly, future studies investigating the moderating role of age in humans should carefully consider the impact of psychiatric comorbidities. While adolescence into young adulthood is the period in which mental health issues often emerge [ 189 , 190 ], there is some evidence that the prevalence of comorbidities is higher in adults with AUD [ 95 ]. This is an important to control for when considering age-related differences on cognition and the brain given the evidence of altered cognitive functioning in other common mental illnesses [ 191 , 192 ].
Concluding remarks
The aim of this systematic review was to extend our understanding of adolescent risk and resilience to the effects of alcohol on brain and cognitive outcomes compared to adults. In comparison to recent existing reviews on the impact of alcohol on the adolescent brain and cognition [ 17 , 18 , 19 , 22 , 23 ], a strength of the current review is the direct comparison of the effects of chronic alcohol exposure during adolescence versus adulthood. This approach allows us to uncover both similarities and differences in the processes underlying alcohol use and dependence between adolescents and adults. However, due to the large degree of heterogeneity in the studies included in sample, designs, and outcomes, we were unable to perform meta-analytic synthesis techniques.
In conclusion, while the identified studies used varying paradigms and outcomes, key patterns of results emerged indicating a complex role of age, with evidence pointing towards both adolescent vulnerability and resilience. The evidence suggests adolescents may be more vulnerable than adults in domains that may promote heavy and binge drinking, including reduced sensitivity to aversive effects of high alcohol dosages, reduced dopaminergic neurotransmission in the NAc and PFC, greater neurodegeneration and impaired neurogenesis, and other neuromodulatory processes. At the same time, adolescents may be more resilient than adults to alcohol-induced impairments in domains which may promote recovery from heavy drinking, such as cognitive flexibility. However, in most domains, the evidence was too limited or inconsistent to draw clear conclusions. Importantly, human studies directly comparing adolescents and adults are largely missing. Recent reviews of longitudinal human research in adolescents, however, revealed consistent evidence of alterations to gray matter, and to a lesser extent white matter, structure in drinkers [ 17 , 18 ], but also highlight the limited evidence available in the domains of neural and cognitive functioning in humans [ 17 ]. Future results from ongoing large-scale longitudinal neuroimaging studies like the ABCD study [ 193 ] will likely shed valuable light on the impact of alcohol use on the adolescent brain. However, our results also stress the need for direct comparisons with adult populations. Moreover, while the lack of experimental control and methodological constraints limit interpretations and causal attributions in human research, translational work aimed at connecting findings from animal models to humans is necessary to build upon the current knowledge base. Furthermore, the use of voluntary self-administration paradigms and incorporation of individual differences and environmental contexts are important steps forward in improving the validity of animal models of alcohol use and related problems. A more informed understanding of the effects of alcohol on adolescents compared to adults can further prevention efforts and better inform policy efforts aimed at minimizing harm during a crucial period for both social and cognitive development.
Degenhardt L, Charlson F, Ferrari A, Santomauro D, Erskine H, Mantilla-Herrara A, et al. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2018;5:987–1012.
Article Google Scholar
Kohn R, Saxena S, Levav I, Saraceno B. The treatment gap in mental health care. World Health Organization; 2004. https://doi.org/10.1590/S0042-96862004001100011 .
Fleury MJ, Djouini A, Huỳnh C, Tremblay J, Ferland F, Ménard JM, et al. Remission from substance use disorders: a systematic review and meta-analysis. Drug Alcohol Depend. 2016;168:293–306.
Article PubMed Google Scholar
Hingson RW, Heeren T, Winter MR. Age of alcohol-dependence onset: associations with severity of dependence and seeking treatment. Pediatrics. 2006;118:e755–e763.
Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006;160:739–46.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th edn. Arlington, VA: American Psychiatric Association; 2013. https://doi.org/10.1176/appi.books.9780890425596.dsm04 .
Conrod P, Nikolaou K. Annual Research Review: On the developmental neuropsychology of substance use disorders. J Child Psychol Psychiatry Allied Discip. 2016;57:371–94.
Spear LP. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol Behav. 2015;148:122–30.
Article CAS PubMed PubMed Central Google Scholar
Simon NW, Gregory TA, Wood J, Moghaddam B. Differences in response initiation and behavioral flexibility between adolescent and adult rats. Behav Neurosci. 2013;127:23–32.
Article PubMed PubMed Central Google Scholar
Carroll LJ, Cassidy JD, Peloso PM, Borg J, von Holst H, Holm L, et al. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2004(Suppl. 43):84–105.
Mastwal S, Ye Y, Ren M, Jimenez DV, Martinowich K, Gerfen CR, et al. Phasic dopamine neuron activity elicits unique mesofrontal plasticity in adolescence. J Neurosci. 2014;34:9484–96.
Article PubMed PubMed Central CAS Google Scholar
Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci. 2012;13:636–50.
Article CAS PubMed Google Scholar
Vergés A, Haeny AM, Jackson KM, Bucholz KK, Grant JD, Trull TJ, et al. Refining the notion of maturing out: results from the national epidemiologic survey on alcohol and related conditions. Am J Public Health. 2013;103:e67–e73.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology 2010;35:217–38.
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56.
Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–9.
de Goede J, van der Mark-Reeuwijk KG, Braun KP, le Cessie S, Durston S, Engels RCME, et al. Alcohol and brain development in adolescents and young adults: a systematic review of the literature and advisory report of the health council of the Netherlands. Adv Nutr. 2021. https://doi.org/10.1093/advances/nmaa170 .
Spear LP. Effects of adolescent alcohol consumption on the brain and behaviour. Nat Rev Neurosci. 2018;19:197–214.
Carbia C, López-Caneda E, Corral M, Cadaveira F. A systematic review of neuropsychological studies involving young binge drinkers. Neurosci Biobehav Rev. 2018;90:332–49.
Feldstein Ewing SW, Sakhardande A, Blakemore SJ. The effect of alcohol consumption on the adolescent brain: a systematic review of MRI and fMRI studies of alcohol-using youth. NeuroImage Clin. 2014;5:420–37.
Article PubMed Central Google Scholar
Squeglia LM, Boissoneault J, Van Skike CE, Nixon SJ, Matthews DB. Age-related effects of alcohol from adolescent, adult, and aged populations using human and animal models. Alcohol Clin Exp Res. 2014;38:2509–16.
Lees B, Meredith LR, Kirkland AE, Bryant BE, Squeglia LM. Effect of alcohol use on the adolescent brain and behavior. Pharm Biochem Behav. 2020;192:172906.
Article CAS Google Scholar
Lees B, Mewton L, Stapinski LA, Squeglia LM, Rae CD, Teesson M. Neurobiological and cognitive profile of young binge drinkers: a systematic review and meta-analysis. Neuropsychol Rev. 2019;29:357–85.
Cservenka A, Brumback T. The burden of binge and heavy drinking on the brain: effects on adolescent and young adult neural structure and function. Front Psychol. 2017;8:1111.
Welch KA, Carson A, Lawrie SM. Brain structure in adolescents and young adults with alcohol problems: systematic review of imaging studies. Alcohol Alcohol. 2013;48:433–44.
Maeda K-I, Satoshi O, Hiroko T. Physiology of reproduction. Academic Press; 2000.
Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63.
Burke AR, Miczek KA. Stress in adolescence and drugs of abuse in rodent models: role of dopamine, CRF, and HPA axis. Psychopharmacology. 2014;231:1557–80.
Doremus-Fitzwater TL, Spear LP. Reward-centricity and attenuated aversions: an adolescent phenotype emerging from studies in laboratory animals. Neurosci Biobehav Rev. 2016;70:121–34.
Rajendran P, Spear LP. The effects of ethanol on spatial and nonspatial memory in adolescent and adult rats studied using an appetitive paradigm. In: Annals of the New York Academy of Sciences. New York Academy of Sciences; 2004. p. 441–4.
Morales M, Schatz KC, Anderson RI, Spear LP, Varlinskaya EI. Conditioned taste aversion to ethanol in a social context: impact of age and sex. Behav Brain Res. 2014;261:323–7.
Dumontheil I. Adolescent brain development. Curr Opin Behav Sci. 2016;10:39–44.
Shillington AM, Woodruff SI, Clapp JD, Reed MB, Lemus H. Self-reported age of onset and telescoping for cigarettes, alcohol, and marijuana: across eight years of the national longitudinal survey of youth. J Child Adolesc Subst Abus. 2012;21:333–48.
Livingston MD, Xu X, Komro KA. Predictors of recall error in self-report of age at alcohol use onset. J Stud Alcohol Drugs. 2016;77:811–8.
De Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31.
Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–91.
Rodriguiz RM, Wetsel WC. Assessments of cognitive deficits in mutant mice. In: Levin ED, Buccafusco JJ, editors. Animal models of cognitive impairment. CRC Press; 2006. p. 223–82.
Leung RK, Toumbourou JW, Hemphill SA. The effect of peer influence and selection processes on adolescent alcohol use: a systematic review of longitudinal studies. Health Psychol Rev. 2014;8:426–57.
Cousijn J, Luijten M, Feldstein Ewing SW. Adolescent resilience to addiction: a social plasticity hypothesis. Lancet Child Adolesc Heal. 2018;2:69–78.
Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149–71.
Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–46.
Vanderschuren LJMJ, Pierce RC. Sensitization processes in drug addiction. Curr Top Behav Neurosci. 2010;3:179–95.
Gorey C, Kuhns L, Smaragdi E, Kroon E, Cousijn J. Age-related differences in the impact of cannabis use on the brain and cognition: a systematic review. Eur Arch Psychiatry Clin Neurosci. 2019;269:37–58.
Schweizer TA, Vogel-Sprott M, Danckert J, Roy EA, Skakum A, Broderick CE. Neuropsychological profile of acute alcohol intoxication during ascending and descending blood alcohol concentrations. Neuropsychopharmacology. 2006;31:1301–9.
Ambrose ML, Bowden SC, Whelan G. Working memory impairments in alcohol-dependent participants without clinical amnesia. Alcohol Clin Exp Res. 2001;25:185–91.
Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol. 2013;18:203–13.
Spear LP. Adolescent neurobehavioral characteristics, alcohol sensitivities, and intake: setting the stage for alcohol use disorders? Child Dev Perspect. 2011;5:231–8.
Holstein SE, Spanos M, Hodge CW. Adolescent C57BL/6J mice show elevated alcohol intake, but reduced taste aversion, as compared to adult mice: a potential behavioral mechanism for binge drinking. Alcohol Clin Exp Res. 2011;35:1842–51.
Moore EM, Forrest RD, Boehm SL. Genotype modulates age-related alterations in sensitivity to the aversive effects of ethanol: an eight inbred strain analysis of conditioned taste aversion. Genes, Brain Behav. 2013;12:70–77.
Schramm-Sapyta NL, DiFeliceantonio AG, Foscue E, Glowacz S, Haseeb N, Wang N, et al. Aversive effects of ethanol in adolescent versus adult rats: potential causes and implication for future drinking. Alcohol Clin Exp Res. 2010;34:2061–9.
Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Ethanol induces second-order aversive conditioning in adolescent and adult rats. Alcohol. 2011;45:45–55.
Carrara-Nascimento PF, Olive MF, Camarini R. Ethanol pre-exposure during adolescence or adulthood increases ethanol intake but ethanol-induced conditioned place preference is enhanced only when pre-exposure occurs in adolescence. Dev Psychobiol. 2014;56:36–48.
Leichtweis KS, Carvalho M, Morais-Silva G, Marin MT, Amaral VCS. Short and prolonged maternal separation impacts on ethanol-related behaviors in rats: sex and age differences. Stress. 2020;23:162–73.
Pautassi RM, Suárez AB, Hoffmann LB, Rueda AV, Rae M, Marianno P, et al. Effects of environmental enrichment upon ethanol-induced conditioned place preference and pre-frontal BDNF levels in adolescent and adult mice. Sci Rep. 2017;7:1–12.
Pascual M, Do Couto BR, Alfonso-Loeches S, Aguilar MA, Rodriguez-Arias M, Guerri C. Changes in histone acetylation in the prefrontal cortex of ethanol-exposed adolescent rats are associated with ethanol-induced place conditioning. Neuropharmacology. 2012;62:2309–19.
Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–88.
Antoniadis EA, McDonald RJ. Amygdala, hippocampus and discriminative fear conditioning to context. Behav Brain Res. 2000;108:1–19.
Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Büchel C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J Neurosci. 2008;28:9030–6.
Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–802.
Quirk GJ, Garcia R, González-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–43.
Bergstrom HC, McDonald CG, Smith RF. Alcohol exposure during adolescence impairs auditory fear conditioning in adult Long-Evans rats. Physiol Behav. 2006;88:466–72.
Broadwater M, Spear LP. Consequences of ethanol exposure on cued and contextual fear conditioning and extinction differ depending on timing of exposure during adolescence or adulthood. Behav Brain Res. 2013;256:10–19.
Broadwater M, Spear LP. Consequences of adolescent or adult ethanol exposure on tone and context fear retention: effects of an acute ethanol challenge during conditioning. Alcohol Clin Exp Res. 2014;38:1454–60.
Broadwater M, Spear LP. Tone conditioning potentiates rather than overshadows context fear in adult animals following adolescent ethanol exposure. Dev Psychobiol. 2014;56:1150–5.
Lacaille H, Duterte-Boucher D, Liot D, Vaudry H, Naassila M, Vaudry D. Comparison of the deleterious effects of binge drinking-like alcohol exposure in adolescent and adult mice. J Neurochem. 2015;132:629–41.
Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. In: Chapple L, editors. Alcoholism: clinical and Experimental Research. Blackwell Publishing Ltd; 1998. p. 416–21.
Acheson SK, Ross EL, Swartzwelder HS. Age-independent and dose-response effects of ethanol on spatial memory in rats. Alcohol. 2001;23:167–75.
Sircar R, Sircar D. Adolescent rats exposed to repeated ethanol treatment show lingering behavioral impairments. Alcohol Clin Exp Res. 2005;29:1402–10.
Swartzwelder HS, Hogan A, Risher ML, Swartzwelder RA, Wilson WA, Acheson SK. Effect of sub-chronic intermittent ethanol exposure on spatial learning and ethanol sensitivity in adolescent and adult rats. Alcohol. 2014;48:353–60.
Matthews DB, Watson MR, James K, Kastner A, Schneider A, Mittleman G. The impact of low to moderate chronic intermittent ethanol exposure on behavioral endpoints in aged, adult, and adolescent rats. Alcohol. 2019;78:33–42.
Galaj E, Barrera E, Morris D, Ma YY, Ranaldi R. Aberrations in incentive learning and responding to heroin in male rats after adolescent or adult chronic binge-like alcohol exposure. Alcohol Clin Exp Res. 2020;44:1214–23.
Diamond A. Executive functions. 2013;64:135–68. https://doi.org/10.1146/annurev-psych-113011-143750 .
Funahashi S, Andreau JM. Prefrontal cortex and neural mechanisms of executive function. J Physiol Paris. 2013;107:471–82.
Schindler AG, Tsutsui KT, Clark JJ. Chronic alcohol intake during adolescence, but not adulthood, promotes persistent deficits in risk-based decision making. Alcohol Clin Exp Res. 2014;38:1622–9.
Risher ML, Fleming RL, Boutros N, Semenova S, Wilson WA, Levin ED, et al. Long-term effects of chronic intermittent ethanol exposure in adolescent and adult rats: radial-arm maze performance and operant food reinforced responding. PLoS ONE. 2013;8:e62940.
Pickens CL, Cook A, Gaeddert B. Dose-dependent effects of alcohol injections on omission-contingency learning have an inverted-U pattern. Behav Brain Res. 2020;392:112736.
Pickens CL, Kallenberger P, Pajser A, Fisher H. Voluntary alcohol access during adolescence/early adulthood, but not during adulthood, causes faster omission contingency learning. Behav Brain Res. 2019;370:111918.
Mejia-Toiber J, Boutros N, Markou A, Semenova S. Impulsive choice and anxiety-like behavior in adult rats exposed to chronic intermittent ethanol during adolescence and adulthood. Behav Brain Res. 2014;266:19–28.
Fernandez GM, Lew BJ, Vedder LC, Savage LM. Chronic intermittent ethanol exposure leads to alterations in brain-derived neurotrophic factor within the frontal cortex and impaired behavioral flexibility in both adolescent and adult rats. Neuroscience. 2017;348:324–34.
Fernandez GM, Stewart WN, Savage LM. Chronic drinking during adolescence predisposes the adult rat for continued heavy drinking: neurotrophin and behavioral adaptation after long-term, continuous ethanol exposure. PLoS ONE 2016;11. https://doi.org/10.1371/journal.pone.0149987 .
Labots M, Cousijn J, Jolink LA, Leon Kenemans J, Vanderschuren LJMJ, Lesscher HMB. Age-related differences in alcohol intake and control over alcohol seeking in rats. Front Psychiatry. 2018;9:419.
Slawecki CJ, Ehlers CL. Enhanced prepulse inhibition following adolescent ethanol exposure in Sprague-Dawley rats. Alcohol Clin Exp Res. 2005;29:1829–36.
White AM, Ghia AJ, Levin ED, Scott Swartzwelder H. Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 2000;24:1251–6.
Baddeley A. Working memory. Science. 1992;255:556–9.
Olton DS, Samuelson RJ. Remembrance of places passed: spatial memory in rats. J Exp Psychol Anim Behav Process. 1976;2:97–116.
Deacon RMJ, Rawlins JNP. T-maze alternation in the rodent. Nat Protoc. 2006;1:7–12.
Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78.
Koch M, Schnitzler HU. The acoustic startle response in rats—circuits mediating evocation, inhibition and potentiation. Behav Brain Res. 1997;89:35–49.
Slawecki CJ, Roth J, Gilder A. Neurobehavioral profiles during the acute phase of ethanol withdrawal in adolescent and adult Sprague-Dawley rats. Behav Brain Res. 2006;170:41–51.
Cunha PJ, Nicastri S, de Andrade AG, Bolla KI. The frontal assessment battery (FAB) reveals neurocognitive dysfunction in substance-dependent individuals in distinct executive domains: abstract reasoning, motor programming, and cognitive flexibility. Addict Behav. 2010;35:875–81.
Jupp B, Dalley JW. Convergent pharmacological mechanisms in impulsivity and addiction: insights from rodent models. Br J Pharm. 2014;171:4729–66.
Dickinson A, Balleine B. Motivational control of goal-directed action. Anim Learn Behav. 1994;22:1–18.
Tomie A, Sharma N. Pavlovian sign-tracking model of alcohol abuse. Curr Drug Abus Rev. 2013;6:201–19.
Tomie A, Jeffers P, Zito B. Sign-tracking model of the addiction blind spot. In: Tomie JMA, editors. Sign tracking and drug addiction. Maize Books; 2018. p. 8–34.
Castillo-Carniglia A, Keyes KM, Hasin DS, Cerdá M. Psychiatric comorbidities in alcohol use disorder. Lancet Psychiatry. 2019;6:1068–80.
Wolitzky-Taylor K, Bobova L, Zinbarg RE, Mineka S, Craske MG. Longitudinal investigation of the impact of anxiety and mood disorders in adolescence on subsequent substance use disorder onset and vice versa. Addict Behav. 2012;37:982–5.
Park J, Moghaddam B. Impact of anxiety on prefrontal cortex encoding of cognitive flexibility. Neuroscience 2017;345:193–202.
Vytal KE, Cornwell BR, Letkiewicz AM, Arkin NE, Grillon C. The complex interaction between anxiety and cognition: Insight from spatial and verbal working memory. Front Hum Neurosci. 2013;7:93.
Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33.
Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67.
Elsey JWB, Kindt M. Startle reflex. In: Zeigler-Hill V, Shackelford TK, editors. Encyclopedia of personality and individual differences. Springer International Publishing; 2018. p. 1–5.
Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharm Biochem Behav. 1980;13:167–70.
File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharm. 2003;463:35–53.
Misslin R, Ropartz P. Responses in mice to a novel object author. Behavior. 1981;78:169–77.
Njung’e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharm Biochem Behav. 1991;38:63–67.
Lee KM, Coelho MA, McGregor HA, Solton NR, Cohen M, Szumlinski KK. Adolescent mice are resilient to alcohol withdrawal-induced anxiety and changes in indices of glutamate function within the nucleus accumbens. Front Cell Neurosci. 2016;10:265.
Agoglia AE, Holstein SE, Reid G, Hodge CW. CaMKIIα-GluA1 activity underlies vulnerability to adolescent binge alcohol drinking. Alcohol Clin Exp Res. 2015;39:1680–90.
Van Skike CE, Diaz-Granados JL, Matthews DB. Chronic intermittent ethanol exposure produces persistent anxiety in adolescent and adult rats. Alcohol Clin Exp Res. 2015;39:262–71.
Conrad KL, Winder DG. Altered anxiety-like behavior and long-term potentiation in the bed nucleus of the stria terminalis in adult mice exposed to chronic social isolation, unpredictable stress, and ethanol beginning in adolescence. Alcohol. 2011;45:585–93.
Slawecki CJ, Roth J. Comparison of the onset of hypoactivity and anxiety-like behavior during alcohol withdrawal adolescent and adult rats. Alcohol Clin Exp Res. 2004;28:598–607.
Wille-Bille A, de Olmos S, Marengo L, Chiner F, Pautassi RM. Long-term ethanol self-administration induces ΔFosB in male and female adolescent, but not in adult, Wistar rats. Prog Neuro-Psychopharmacol Biol Psychiatry. 2017;74:15–30.
Varlinskaya EI, Spear LP. Chronic tolerance to the social consequences of ethanol in adolescent and adult Sprague-Dawley rats. Neurotoxicol Teratol. 2007;29:23–30.
Morales M, Varlinskaya EI, Spear LP. Age differences in the expression of acute and chronic tolerance to ethanol in male and female rats. Alcohol Clin Exp Res. 2011;35:1614–24.
PubMed PubMed Central Google Scholar
Neuhofer D, Kalivas P. Metaplasticity at the addicted tetrapartite synapse: a common denominator of drug induced adaptations and potential treatment target for addiction. Neurobiol Learn Mem. 2018;154:97–111.
Pian JP, Criado JR, Milner R, Ehlers CL. N-methyl-d-aspartate receptor subunit expression in adult and adolescent brain following chronic ethanol exposure. Neuroscience. 2010;170:645–54.
Falco AM, Bergstrom HC, Bachus SE, Smith RF. Persisting changes in basolateral amygdala mRNAs after chronic ethanol consumption. Physiol Behav. 2009;96:169–73.
Chin VS, Van Skike CE, Berry RB, Kirk RE, Diaz-Granados J, Matthews DB. Effect of acute ethanol and acute allopregnanolone on spatial memory in adolescent and adult rats. Alcohol. 2011;45:473–83.
Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–31.
Akkus F, Mihov Y, Treyer V, Ametamey SM, Johayem A, Senn S, et al. Metabotropic glutamate receptor 5 binding in male patients with alcohol use disorder. Transl Psychiatry 2018;8. https://doi.org/10.1038/s41398-017-0066-6 .
Leurquin-Sterk G, Ceccarini J, Crunelle CL, De Laat B, Verbeek J, Deman S, et al. Lower limbic metabotropic glutamate receptor 5 availability in alcohol dependence. J Nucl Med. 2018;59:682–90.
Davies M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J Psychiatry Neurosci. 2003;28:263–74.
Grobin AC, Matthews DB, Montoya D, Wilson WA, Morrow AL, Swartzwelder HS. Age-related differences in neurosteroid potentiation of muscimol-stimulated 36Cl- flux following chronic ethanol treatment. Neuroscience. 2001;105:547–52.
Fleming RL, Acheson SK, Moore SD, Wilson WA, Swartzwelder HS. GABA transport modulates the ethanol sensitivity of tonic inhibition in the rat dentate gyrus. Alcohol. 2011;45:577–83.
Fleming RL, Li Q, Risher ML, Sexton HG, Moore SD, Wilson WA, et al. Binge-pattern ethanol exposure during adolescence, but not adulthood, causes persistent changes in GABAA receptor-mediated tonic inhibition in dentate granule cells. Alcohol Clin Exp Res. 2013;37:1154–60.
Carrara-Nascimento PF, Hoffmann LB, Flório JC, Planeta CS, Camarini R. Effects of ethanol exposure during adolescence or adulthood on locomotor sensitization and dopamine levels in the reward system. Front Behav Neurosci. 2020;14:31.
Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 2012;76:116–29.
Wu J, Gao M, Taylor DH. Neuronal nicotinic acetylcholine receptors are important targets for alcohol reward and dependence. Acta Pharmacol Sin. 2014;35:311–5.
Walker LC, Berizzi AE, Chen NA, Rueda P, Perreau VM, Huckstep K, et al. Acetylcholine muscarinic M4 receptors as a therapeutic target for alcohol use disorder: converging evidence from humans and rodents. Biol Psychiatry. 2020;88:898–909.
Vetreno RP, Broadwater M, Liu W, Spear LP, Crews FT. Adolescent, but not adult, binge ethanol exposure leads to persistent global reductions of choline acetyltransferase expressing neurons in brain. PLoS ONE. 2014;9:113421.
Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–73.
Huang C, Titus JA, Bell RL, Kapros T, Chen J, Huang R. A mouse model for adolescent alcohol abuse: stunted growth and effects in brain. Alcohol Clin Exp Res. 2012;36:1728–37.
Crews FT, Braun CJ, Hoplight B, Switzer RC, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–23.
Broadwater MA, Liu W, Crews FT, Spear LP. Persistent loss of hippocampal neurogenesis and increased cell death following adolescent, but not adult, chronic ethanol exposure. Dev Neurosci. 2014;36:297–305.
Nixon K, Kim DH, Potts EN, He J, Crews FT. Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiol Dis. 2008;31:218–29.
Camp MC, Mayfield RD, McCracken M, McCracken L, Alcantara AA. Neuroadaptations of Cdk5 in cholinergic interneurons of the nucleus accumbens and prefrontal cortex of inbred alcohol-preferring rats following voluntary alcohol drinking. Alcohol Clin Exp Res. 2006;30:1322–35.
Goulding SP, de Guglielmo G, Carrette LLG, George O, Contet C. Systemic administration of the cyclin-dependent kinase inhibitor (S)-CR8 selectively reduces escalated ethanol intake in dependent rats. Alcohol Clin Exp Res. 2019;43:2079–89.
Joe KH, Kim YK, Kim TS, Roh SW, Choi SW, Kim YB, et al. Decreased plasma brain-derived neurotrophic factor levels in patients with alcohol dependence. Alcohol Clin Exp Res. 2007;31:1833–8.
Huang MC, Chen CH, Chen CH, Liu SC, Ho CJ, Shen WW, et al. Alterations of serum brain-derived neurotrophic factor levels in early alcohol withdrawal. Alcohol Alcohol. 2008;43:241–5.
Miller R, King MA, Heaton MB, Walker DW. The effects of chronic ethanol consumption on neurotrophins and their receptors in the rat hippocampus and basal forebrain. Brain Res. 2002;950:137–47.
Vetreno RP, Crews FT. Binge ethanol exposure during adolescence leads to a persistent loss of neurogenesis in the dorsal and ventral hippocampus that is associated with impaired adult cognitive functioning. Front Neurosci. 2015;9:35.
Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–37.
Faria RR, Lima Rueda AV, Sayuri C, Soares SL, Malta MB, Carrara-Nascimento PF, et al. Environmental modulation of ethanol-induced locomotor activity: Correlation with neuronal activity in distinct brain regions of adolescent and adult Swiss mice. Brain Res. 2008;1239:127–40.
Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, et al. Cytokines and alcohol. In: Alcoholism: clinical and experimental research. John Wiley & Sons, Ltd; 2006. p. 720–30.
Davis RL, Syapin PJ. Chronic ethanol inhibits CXC chemokine ligand 10 production in human A172 astroglia and astroglial-mediated leukocyte chemotaxis. Neurosci Lett. 2004;362:220–5.
Knapp DJ, Crews FT. Induction of cyclooxygenase-2 in brain during acute and chronic ethanol treatment and ethanol withdrawal. Alcohol Clin Exp Res. 1999;23:633–43.
Marshall SA, McClain JA, Wooden JI, Nixon K. Microglia dystrophy following binge-like alcohol exposure in adolescent and adult male rats. Front Neuroanat. 2020;14:52.
Agrawal RG, Owen JA, Levin PS, Hewetson A, Berman AE, Franklin SR, et al. Bioinformatics analyses reveal age-specific neuroimmune modulation as a target for treatment of high ethanol drinking. Alcohol Clin Exp Res. 2014;38:428–37.
Kane CJM, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J, et al. Effects of ethanol on immune response in the brain: region-specific changes in adolescent versus adult mice. Alcohol Clin Exp Res. 2014;38:384–91.
Blaine SK, Sinha R. Alcohol, stress, and glucocorticoids: from risk to dependence and relapse in alcohol use disorders. Neuropharmacology 2017;122:136–47.
Slawecki CJ, Jiménez-Vasquez P, Mathé AA, Ehlers CL. Effect of ethanol on brain neuropeptides in adolescent and adult rats. J Stud Alcohol. 2005;66:46–52.
van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76:98–115.
Souza-Moreira L, Campos-Salinas J, Caro M, Gonzalez-Rey E. Neuropeptides as pleiotropic modulators of the immune response. Neuroendocrinology. 2011;94:89–100.
Carniglia L, Ramírez D, Durand D, Saba J, Turati J, Caruso C, et al. Neuropeptides and microglial activation in inflammation, pain, and neurodegenerative diseases. Mediators Inflamm. 2017;2017. https://doi.org/10.1155/2017/5048616 .
Hipolito L, Sanchez M, Polache A, Granero L. Brain metabolism of ethanol and alcoholism: an update. Curr Drug Metab. 2007;8:716–27.
Rhoads DE, Contreras C, Fathalla S. Brain levels of catalase remain constant through strain, developmental, and chronic alcohol challenges. Enzyme Res. 2012;2012. https://doi.org/10.1155/2012/572939 .
Vasiliou V, Ziegler TL, Bludeau P, Petersen DR, Gonzalez FJ, Deitrich RA. CYP2E1 and catalase influence ethanol sensitivity in the central nervous system. Pharmacogenet Genomics. 2006;16:51–58.
Zimatkin SM, Buben AI. Ethanol oxidation in the living brain. Alcohol Alcohol. 2007;42:529–32.
Hargreaves GA, Quinn H, Kashem MA, Matsumoto I, McGregor IS. Proteomic analysis demonstrates adolescent vulnerability to lasting hippocampal changes following chronic alcohol consumption. Alcohol Clin Exp Res. 2009;33:86–94.
Galaj E, Guo C, Huang D, Ranaldi R, Ma YY. Contrasting effects of adolescent and early-adult ethanol exposure on prelimbic cortical pyramidal neurons. Drug Alcohol Depend. 2020;216:108309.
Li Q, Fleming RL, Acheson SK, Madison RD, Moore SD, Risher ML, et al. Long-term modulation of A-type K+ conductances in hippocampal CA1 interneurons in rats after chronic intermittent ethanol exposure during adolescence or adulthood. Alcohol Clin Exp Res. 2013;37:2074–85.
Artinian J, Lacaille JC. Disinhibition in learning and memory circuits: new vistas for somatostatin interneurons and long-term synaptic plasticity. Brain Res Bull. 2018;141:20–26.
Müller-Oehring EM, Kwon D, Nagel BJ, Sullivan EV, Chu W, Rohlfing T, et al. Influences of age, sex, and moderate alcohol drinking on the intrinsic functional architecture of adolescent brains. Cereb Cortex. 2018;28:1049–63.
McAteer AM, Hanna D, Curran D. Age-related differences in alcohol attention bias: a cross-sectional study. Psychopharmacology. 2018;235:2387–93.
Rooke SE, Hine DW. A dual process account of adolescent and adult binge drinking. Addict Behav. 2011;36:341–6.
Cousijn J, Green KH, Labots M, Vanderschuren LJMJ, Kenemans JL, Lesscher HMB. Motivational and control mechanisms underlying adolescent versus adult alcohol use. NeuroSci. 2020;1:44–58.
Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20.
Cousijn J, van Benthem P, van der Schee E, Spijkerman R. Motivational and control mechanisms underlying adolescent cannabis use disorders: a prospective study. Dev Cogn Neurosci. 2015;16:36–45.
Saalfield J, Spear L. Consequences of repeated ethanol exposure during early or late adolescence on conditioned taste aversions in rats. Dev Cogn Neurosci. 2015;16:174–82.
Saalfield J, Spear L. The ontogeny of ethanol aversion. Physiol Behav. 2016;156:164–70.
McQuown SC, Wood MA. Epigenetic regulation in substance use disorders. Curr Psychiatry Rep. 2010;12:145–53.
Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–50.
Logrip ML, Barak S, Warnault V, Ron D. Corticostriatal BDNF and alcohol addiction. Brain Res. 2015;1628:60–67.
Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience 2012;215:42–58.
Robinson OJ, Pike AC, Cornwell B, Grillon C. The translational neural circuitry of anxiety. J Neurol Neurosurg Psychiatry. 2019;90:1353–60.
PubMed Google Scholar
Martínez G, Ropero C, Funes A, Flores E, Blotta C, Landa AI, et al. Effects of selective NMDA and non-NMDA blockade in the nucleus accumbens on the plus-maze test. Physiol Behav. 2002;76:219–24.
Bergink V, Van Megen HJGM, Westenberg HGM. Glutamate and anxiety. Eur Neuropsychopharmacol. 2004;14:175–83.
Scott AJ, Jordan M, Lueras BJN. Effects of binge drinking on the developing brain. Alcohol Res. 2018;39:87–96.
Google Scholar
Krieger H, Young CM, Anthenien AM, Neighbors C. The epidemiology of binge drinking among college-age individuals in the United States. Alcohol Res. 2018;39:23–30.
Hooijmans CR, Rovers MM, De Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:1–9.
Krauth D, Woodruff TJ, Bero L. Instruments for assessing risk of bias and other methodological criteria of published animal studies: a systematic review. Environ Health Perspect. 2013;121:985–92.
Field M, Kersbergen I. Are animal models of addiction useful? Addiction. 2020;115:6–12.
Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–23.
Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63.
Spoelder M, Hesseling P, Baars AM, Lozeman-van’t Klooster JG, Rotte MD, Vanderschuren LJMJ, et al. Individual variation in alcohol intake predicts reinforcement, motivation, and compulsive alcohol use in rats. Alcohol Clin Exp Res. 2015;39:2427–37.
Fredriksson I, Venniro M, Reiner DJ, Chow JJ, Bossert JM, Shaham Y. Animal models of drug relapse and craving after voluntary abstinence: a review. Pharm Rev. 2021;73:1050–83.
Vanderschuren LJMJ, Ahmed SH. Animal models of the behavioral symptoms of substance use disorders. Cold Spring Harb Perspect Med. 2021;11:a040287.
Kuhn BN, Kalivas PW, Bobadilla AC. Understanding addiction using animal models. Front Behav Neurosci. 2019;13. https://doi.org/10.3389/fnbeh.2019.00262 .
Venniro M, Shaham Y. An operant social self-administration and choice model in rats. Nat Protoc. 2020;15:1542–59.
Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. Lancet 2012;379:1056–67.
Costello EJ, Egger H, Angold A. 10-Year research update review: the epidemiology of child and adolescent psychiatric disorders: I. Methods and public health burden. J Am Acad Child Adolesc Psychiatry. 2005;44:972–86.
Snyder HR, Kaiser RH, Whisman MA, Turner AEJ, Guild RM, Munakata Y. Opposite effects of anxiety and depressive symptoms on executive function: the case of selecting among competing options. 2014;28:893–902. https://doi.org/10.1080/026999312013859568 .
McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119:1–8.
Volkow ND, Koob GF, Croyle RT, Bianchi DW, Gordon JA, Koroshetz WJ, et al. The conception of the ABCD study: from substance use to a broad NIH collaboration. Dev Cogn Neurosci. 2018;32:4–7.
Download references
Acknowledgements
This work was supported by grant 1RO1 DA042490-01A1 awarded to Janna Cousijn and Francesca Filbey from the National Institute on Drug Abuse/National Institutes of Health. The grant supported the salaries of authors Lauren Kuhns, Emese Kroon, and Janna Cousijn. Thank you to Claire Gorey (CG) for running the initial search and aiding in the screening process.
Author information
Authors and affiliations.
Neuroscience of Addiction (NofA) Lab, Department of Psychology, University of Amsterdam, Amsterdam, The Netherlands
Lauren Kuhns, Emese Kroon, Gabry Mies & Janna Cousijn
The Amsterdam Brain and Cognition Center (ABC), University of Amsterdam, Amsterdam, The Netherlands
Lauren Kuhns, Emese Kroon & Janna Cousijn
Department of Animals in Science and Society, Division of Behavioural Neuroscience, Faculty of Veterinary Medicine, Utrecht University, Utrecht, The Netherlands
Heidi Lesscher
Department of Psychology, Education & Child Studies, Erasmus University Rotterdam, Rotterdam, The Netherlands
Janna Cousijn
You can also search for this author in PubMed Google Scholar
Contributions
LK conducted the systematic searches; LK, EK, GM, and JC screened the citations for exclusion and inclusion; LK, EK, HL, and JC wrote the review; LK, EK, HL, GM, and JC revised the manuscript.
Corresponding author
Correspondence to Lauren Kuhns .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kuhns, L., Kroon, E., Lesscher, H. et al. Age-related differences in the effect of chronic alcohol on cognition and the brain: a systematic review. Transl Psychiatry 12 , 345 (2022). https://doi.org/10.1038/s41398-022-02100-y
Download citation
Received : 26 October 2021
Revised : 21 June 2022
Accepted : 28 July 2022
Published : 25 August 2022
DOI : https://doi.org/10.1038/s41398-022-02100-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Emotional and cognitive influences on alcohol consumption in middle-aged and elderly tanzanians: a population-based study.
- Patrick Kazonda
- Till Bärnighausen
Scientific Reports (2024)
Ethanol’s impact on the brain: a neurobiological perspective on the mechanisms of memory impairment
- Mahdiyeh Hedayati-Moghadam
- Fateme Razazpour
- Yousef Baghcheghi
Molecular Biology Reports (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Observatory
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Fact sheets /
- Alcohol or alcoholic beverages contain ethanol, a psychoactive and toxic substance that can cause dependence.
- Worldwide, around 2.6 million deaths were caused by alcohol consumption in 2019. Of these, 1.6 million deaths were from noncommunicable diseases, 700 000 deaths from injuries and 300 000 deaths from communicable diseases.
- The alcohol-attributable mortality was heaviest among men, accounting for 2 million deaths compared to 600 000 deaths among women, in 2019.
- An estimated 400 million people, or 7% of the world’s population aged 15 years and older, lived with alcohol use disorders. Of this, 209 million people (3.7% of the adult world population) lived with alcohol dependence.
- Alcohol consumption, even at low levels can bring health risks, but most alcohol related harms come from heavy episodic or heavy continuous alcohol consumption.
- Effective alcohol control interventions exist and should be utilized more, at the same time it is important for people to know risks associated with alcohol consumption and take individual actions to protect from its harmful effects.
Alcohol and alcoholic beverages contain ethanol, which is a psychoactive and toxic substance with dependence-producing properties. Alcohol has been widely used in many cultures for centuries, but it is associated with significant health risks and harms.
Worldwide, 2.6 million deaths were attributable to alcohol consumption in 2019, of which 2 million were among men and 0.6 million among women. The highest levels of alcohol-related deaths per 100 000 persons are observed in the WHO European and African Regions with 52.9 deaths and 52.2 deaths per 100 000 people, respectively.
People of younger age (20–39 years) are disproportionately affected by alcohol consumption with the highest proportion (13%) of alcohol-attributable deaths occurring within this age group in 2019.
The data on global alcohol consumption in 2019 shows that an estimated 400 million people aged 15 years and older live with alcohol use disorders, and an estimated 209 million live with alcohol dependence.
There has been some progress; from 2010 to 2019, the number of alcohol-attributable deaths per 100 000 people decreased by 20.2% globally.
There has been a steady increase in the number of countries developing national alcohol policies. Almost all countries implement alcohol excise taxes. However, countries report continued interference from the alcohol industry in policy development.
Based on 2019 data, about 54% out of 145 reporting countries had national guidelines/standards for specialized treatment services for alcohol use disorders, but only 46% of countries had legal regulations to protect the confidentiality of people in treatment.
Access to screening, brief intervention and treatment for people with hazardous alcohol use and alcohol use disorder remains very low, as well as access to medications for treatment of alcohol use disorders. Overall, the proportion of people with alcohol use disorders in contact with treatment services varies from less than 1% to no more than 14% in all countries where such data are available.
Health risks of alcohol use
Alcohol consumption is found to play a causal role in more than 200 diseases, injuries and other health conditions. However, the global burden of disease and injuries caused by alcohol consumption can be quantified for only 31 health conditions on the basis of the available scientific evidence for the role of alcohol use in their development, occurrence and outcomes.
Drinking alcohol is associated with risks of developing noncommunicable diseases such as liver diseases, heart diseases, and different types of cancers, as well as mental health and behavioural conditions such as depression, anxiety and alcohol use disorders.
An estimated 474 000 deaths from cardiovascular diseases were caused by alcohol consumption in 2019.
Alcohol is an established carcinogen and alcohol consumption increases the risk of several cancers, including breast, liver, head and neck, oesophageal and colorectal cancers. In 2019, 4.4% of cancers diagnosed globally and 401 000 cancer deaths were attributed to alcohol consumption.
Alcohol consumption also causes significant harm to others, not just to the person consuming alcohol. A significant part of alcohol-attributable disease burden arises from injuries such as road traffic accidents. In 2019, of a total of 298 000 deaths from alcohol-related road crashes, 156 000 deaths were caused by someone else’s drinking.
Other injuries, intentional or unintentional, include falls, drowning, burns, sexual assault, intimate partner violence and suicide.
A causal relationship has been established between alcohol use and the incidence or outcomes of infectious diseases such as tuberculosis and HIV.
Alcohol consumption during pregnancy increases the risk of having a child with fetal alcohol spectrum disorders (FASDs), the most severe form of which is fetal alcohol syndrome (FAS), which is associated with developmental disabilities and birth defects. Alcohol consumption during pregnancy can also increase the risk of pre-term birth complications including miscarriage, stillbirth and premature delivery.
Younger people are disproportionately negatively affected by alcohol consumption, with the highest proportion (13%) of alcohol-attributable deaths in 2019 occurring among people aged between 20 and 39 years.
In the long term, harmful and hazardous levels of alcohol consumption can lead to social problems including family problems, issues at work, financial problems, and unemployment.
Factors affecting alcohol consumption and alcohol-related harm
There is no form of alcohol consumption that is risk-free. Even low levels of alcohol consumption carry some risks and can cause harm.
The level of risk depends on several factors, including the amount consumed, frequency of drinking, the health status of the individual, age, sex, and other personal characteristics, as well as the context in which alcohol consumption occurs.
Some groups and individuals who are vulnerable or at risk may have a higher susceptibility to the toxic, psychoactive and dependence-inducing properties of alcohol. On the other hand, individuals who adopt lower-risk patterns of alcohol consumption may not necessarily face a significantly increased likelihood of negative health and social consequences.
Societal factors which affect the levels and patterns of alcohol consumption and related problems include cultural and social norms, availability of alcohol, level of economic development, and implementation and enforcement of alcohol policies.
The impact of alcohol consumption on chronic and acute health outcomes is largely determined by the total volume of alcohol consumed and the pattern of drinking, particularly those patterns which are associated with the frequency of drinking and episodes of heavy drinking. Most alcohol related harms come from heavy episodic or heavy continuous alcohol consumption.
The context plays an important role in the occurrence of alcohol-related harm, particularly as a result of alcohol intoxication. Alcohol consumption can have an impact not only on the incidence of diseases, injuries and other health conditions, but also on their outcomes and how these evolve over time.
There are gender differences in both alcohol consumption and alcohol-related mortality and morbidity. In 2019, 52% of men were current drinkers, while only 35% of women had been drinking alcohol in the last 12 months. Alcohol per capita consumption was, on average, 8.2 litres for men compared to 2.2 litres for women. In 2019, alcohol use was responsible for 6.7% of all deaths among men and 2.4% of all deaths among women.
WHO response
The Global alcohol action plan 2022–2030, endorsed by WHO Member States, aims to reduce the harmful use of alcohol through effective, evidence-based strategies at national, regional and global levels. The plan outlines six key areas for action: high-impact strategies and interventions, advocacy and awareness, partnership and coordination, technical support and capacity-building, knowledge production and information systems, and resource mobilization.
Implementation of global strategy and action plan will accelerate global progress towards attaining alcohol-related targets under the Sustainable Development Goal 3.5 on strengthening the prevention and treatment of substance abuse, including narcotic drug abuse and harmful use of alcohol.
Achieving this will require global, regional and national actions on the levels, patterns and contexts of alcohol consumption and the wider social determinants of health, with a particular focus on implementing high-impact cost effective interventions.
It is vital to address the determinants that drive the acceptability, availability and affordability of alcohol consumption through cross-sectoral, comprehensive and integrated policy measures. It is also of critical importance to achieve universal health coverage for people living with alcohol use disorders and other health conditions due to alcohol use by strengthening health system responses and developing comprehensive and accessible systems of treatment and care that for those in need.
The SAFER initiative, launched in 2018 by WHO and partners, supports countries to implement the high-impact, cost-effective interventions proven to reduce the harm caused by alcohol consumption.
The WHO Global Information System on Alcohol and Health (GISAH) presents data on levels and patterns of alcohol consumption, alcohol-attributable health and social consequences and policy responses across the world.
Achieving a reduction in the harmful use of alcohol in line with the targets included in the Global alcohol action plan, the SDG 2030 agenda and the WHO Global monitoring framework for noncommunicable diseases, requires concerted action by countries and effective global governance.
Public policies and interventions to prevent and reduce alcohol-related harm should be guided and formulated by public health interests and based on clear public health goals and the best available evidence.
Engaging all relevant stakeholders is essential but the potential conflicts of interest, particularly with the alcohol industry, must be carefully assessed before engagement. Economic operators should refrain from activities that might prevent, delay or stop the development, enactment, implementation and enforcement of high-impact strategies and interventions to reduce the harmful use of alcohol.
By working together, with due diligence and protection from conflicts of interest, the negative health and social consequences of alcohol can be effectively reduced.
Global status report on alcohol and health and treatment of substance use disorders
Global strategy to reduce the harmful use of alcohol
Global Alcohol Action Plan 2022–2030
SAFER Alcohol Control Initiative
More on alcohol
- Share full article
Advertisement
Supported by
Even a Little Alcohol Can Harm Your Health
Recent research makes it clear that any amount of drinking can be detrimental. Here’s why you may want to cut down on your consumption beyond Dry January.

By Dana G. Smith
Sorry to be a buzz-kill, but that nightly glass or two of wine is not improving your health.
After decades of confusing and sometimes contradictory research (too much alcohol is bad for you but a little bit is good; some types of alcohol are better for you than others; just kidding, it’s all bad), the picture is becoming clearer: Even small amounts of alcohol can have health consequences.
Research published in November revealed that between 2015 and 2019, excessive alcohol use resulted in roughly 140,000 deaths per year in the United States. About 40 percent of those deaths had acute causes, like car crashes, poisonings and homicides. But the majority were caused by chronic conditions attributed to alcohol, such as liver disease, cancer and heart disease.
When experts talk about the dire health consequences linked to excessive alcohol use, people often assume that it’s directed at individuals who have an alcohol use disorder. But the health risks from drinking can come from moderate consumption as well.
“Risk starts to go up well below levels where people would think, ‘Oh, that person has an alcohol problem,’” said Dr. Tim Naimi, director of the University of Victoria’s Canadian Institute for Substance Use Research. “Alcohol is harmful to the health starting at very low levels.”
If you’re wondering whether you should cut back on your drinking, here’s what to know about when and how alcohol impacts your health.
How do I know if I’m drinking too much?
“Excessive alcohol use” technically means anything above the U.S. Dietary Guidelines ’ recommended daily limits. That’s more than two drinks a day for men and more than one drink a day for women.
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .

New insights on college drinking
Psychologists' research is pinpointing who is most at risk for drinking problems in college and developing more targeted, evidence-based interventions.
By Anna Miller
Monitor Staff
October 2013, Vol 44, No. 9
Print version: page 46
13 min read

At the University at Albany in 2000, Chad Waxman fit the profile of a college student primed for risky drinking: A freshman male fraternity brother who drank in high school, Waxman chose Albany in part for its balance between work and play. "I wanted that time to let loose," he says.
Despite the predictors, Waxman sailed through college in health and happiness, even serving in student government and winning multiple leadership awards at the university before graduating in 2003. He went on to earn his master's degree in counseling psychology and school counseling from Albany in 2005 and is now a PsyD candidate at Nova Southeastern University.
How did Waxman, now 33, avoid the pitfalls of drinking common among college students? That's a question psychologists are probing deeply. After all, each year, more than 1,825 college students die from alcohol-related accidents and nearly 600,000 are injured while drunk, according to a 2009 study in the Journal of Studies on Alcohol and Drugs . Another 696,000 are assaulted by another student who has been drinking, and 97,000 are victims of alcohol-related sexual assault or date rape, the study found.
Then there's the 25 percent of college students who report academic consequences related to alcohol — a hangover can quickly derail plans for class or study — and the 11 percent who admit damaging property after a night of drinking ( Journal of American College Health , 2002). An estimated 5 percent get into legal trouble as a result of alcohol, the same study found. In all, of the 80 percent of college students who drink alcohol, half "binge drink," or consume about four drinks in two hours for women and five in two hours for men, according to the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
"College drinking is sometimes still viewed as a harmless rite of passage, when in fact [college students] are drinking more than any other age or demographic group," says psychologist James Murphy, PhD, of the University of Memphis, who studies addictive and health risk behaviors, including among college students.
That's particularly dangerous given that research shows this age group is much more impulsive even when alcohol's not involved, he says. There's also evidence suggesting that excessive alcohol use in young adulthood may impair brain development, including in cognition and memory, according to the NIAAA .
But college also presents an opportune time to equip students with the skills to approach alcohol intelligently, says Murphy. With 63 percent of young Americans ages 25 to 29 having completed at least some college, according to a report from the Pew Research Center, the setting is "a last prevention point for our society to address the risks associated with drinking," he says. (Most research on college drinking so far involves mainly full-time students in four-year colleges and universities.)
For Waxman, the time was ripe. As a peer facilitator in Albany's Counseling Center, he helped motivate other students — and in effect, himself — to shift their drinking behaviors using one of many emerging interventions designed and tested by psychologists. The approaches address why a student drinks and are tailored for specific populations of students, such as athletes and freshmen. Some interventions are targeted to align with specific events, such as 21st birthday celebrations, as a way to reroute dangerous decisions made on a night that notoriously gets out of control.
"Through learning the realities of alcohol, I realized you don't have to drink like it's a competition to have fun," Waxman says.
Most important, these interventions are evidence-based, says Mary Larimer, PhD, director of the University of Washington's Center for the Study of Health and Risk Behaviors and associate director of the Addictive Behaviors Research Center.
"We know a lot more about what influences excessive alcohol use in this population and we can tailor the interventions to address those risk factors as well," Larimer says. "That's contributed to our ability to make a difference."
Prevention efforts
One way psychologists are fine-tuning their efforts is by pinpointing who is most at risk for problems related to drinking. So far, research indicates that those most at risk are incoming freshmen, student athletes and those involved in the Greek system. Studies also show that men tend to drink more on average than women — but women progress faster over time from alcohol use to abuse, says Larimer. In fact, one study led by psychologist Bettina Hoeppner, PhD, of Harvard Medical School's Center for Addiction Medicine, found that college women exceed the NIAAA's weekly limits more often than men ( Alcoholism: Clinical and Experimental Research , 2013).
"The gender gaps have closed a lot," Larimer says.
Personality factors, such as impulsivity and sensation-seeking, also contribute to risky drinking. Psychological research suggests that how different people respond to alcohol can help predict whose behavior will become problematic. Those who need a lot to experience its effects or who experience more of alcohol's stimulating rather than sedative effects, for example, are at higher risk. Students who overestimate how much their peers drink, as well as those who expect great things from alcohol ("I will feel outgoing and meet my future boyfriend!"), are more likely to overindulge and experience alcohol's negative consequences, such as engaging in unsafe sex, adds Larimer.
Another factor appears to distinguish between students who drink a lot yet remain relatively safe and those who drink the same amount or less yet suffer the consequences: subjective intoxication. In other words, a student's likelihood to get into trouble during or after drinking has as much to do with how drunk he or she feels as it does with how much he or she actually drinks, according to an NIAAA-funded study conducted by Kim Fromme, PhD, of the University of Texas at Austin's SAHARA Lab (Studies on Alcohol, Health and Risky Activities) and colleagues. And those different perceptions could have biological roots, Fromme says.
"We're predicting specific genetic influences on those differences in people's subjective levels of intoxication," she says.
Why a student drinks can also reveal a lot about how problematic his or her alcohol use may become, according to Clayton Neighbors, PhD, who directs the University of Houston's Social Influences and Health Behaviors Lab. While some students drink for social and environmental reasons, such as being at a party, others drink for emotional reasons, such as coping with a bad grade or a breakup. It's the latter group — who may be turning to alcohol to handle another mental health problem such as post-traumatic stress disorder, depression or anxiety — whose members are primed for long-term alcohol abuse, researchers say.
More effective interventions
Up until the late 1990s, most colleges and universities approached risky drinking from a one-size-fits-all perspective. Campus-wide awareness campaigns and educational sessions during freshman orientation were popular but ineffective, the NIAAA Task Force on College Drinking found in 2002.
That changed in 1999 when the late psychologist Alan Marlatt, PhD, of the University of Washington, and his team introduced Brief Alcohol Screening and Intervention for College Students, or BASICS. The intervention is used in varying forms by colleges nationwide when students come in for primary care or mental health services or are referred for an alcohol-related offense. BASICS gives students personalized feedback on their drinking behaviors, including comparing how much they drink with how much the average student on their campus drinks. The intervention also uses motivational interviewing by asking students open-ended, non-judgmental questions to explore drinking behaviors and generate motivation to change. Finally, it offers individualized strategies — such as putting ice in drinks or assigning a designated driver — to help students drink in less risky ways. The method, which has been shown to reduce how much students drink as well as to reduce related negative consequences up to four years out, meets NIAAA's highest standards for evidence-based college drinking interventions (American Journal of Public Health, 2001).
But BASICS doesn't work for every student. Those with high levels of social anxiety, for example, aren't easily influenced to change by the notion that they're overestimating how much their peers really drink. This can make them less receptive to the "norms correction" component of BASICS, a 2012 study in Psychology of Addictive Behaviors finds. About one-third of students who receive the intervention don't change their drinking habits. Another drawback to the intervention is staffing: The traditional method requires one or two 50-minute sessions with a trained facilitator, who is often a mental health professional.
That's why many psychologists are experimenting with variations of BASICS, such as by offering it in a Web-based format or presented by trained peers, rather than by mental health professionals. Researchers are also looking at ways to shorten the intervention: A 2013 study in Addictive Behaviors by Larimer and colleagues found that a 10-minute version of BASICS was just as effective as a 50-minute one.
Larimer says shortening the intervention by picking and choosing from among its individual components — namely, the part that corrects students' misperceptions of campus norms and the one that offers strategies for safer drinking — might be enough to elicit short-term effects and to work for students at lower risk. "The more comprehensive interventions, then, may have longer-lasting effects," she suspects, but she says more research is needed to tease apart which variations work for whom.
There's also evidence that students can deliver the interventions just as effectively as mental health professionals. In one study, Larimer and colleagues delivered a BASICS-like intervention to 12 fraternities, varying who gave them feedback — either a peer interviewer or a professional research staffer. They found that both groups significantly reduced their alcohol intake when compared with controls ( Journal of Alcohol Studies , 2001). Another study led by Fromme that looked at peers and professional providers who headed an alcohol prevention "lifestyle management course" for college students found similar outcomes ( Journal of Consulting and Clinical Psychology , 2004).
But the research comes with caveats, says University at Albany psychologist Maria Dolores Cimini, PhD, who explored peer facilitators' effectiveness through a five-year study funded by an NIAAA Rapid Response to College Drinking Problems grant and got mixed results. "Students can deliver these interventions, but they must be well-trained and very closely supervised," she says ( Journal of Studies on Alcohol and Drugs , 2009).
Waxman, who became trained as a peer facilitator at Albany's Counseling Center during his sophomore year, said his efforts paid off among the peers he intervened with. "Having someone you can relate to … saying, ‘This is the reality,' really changes behavior," he says.
Building on BASICS
At the University at Albany Counseling Center, an intervention called the STEPS Comprehensive Alcohol Screening and Brief Intervention Program takes BASICS and tailors it for specific populations of high-risk drinkers, including first-year students, student athletes and students seeking primary health and mental health care on campus. A student athlete, for example, learns how alcohol affects hydration and athletic performance — even days after taking the last sip.
The key is speaking the students' language, says Cimini, who directs the program. "If we can't engage students and get them in for the intervention in the first place, we lose a golden opportunity to mobilize the change process at a time when students are most resilient and receptive to interventions."
In surveys conducted three and six months post-intervention, STEPS has been shown to significantly reduce alcohol use and risky behavior among each subgroup. Colleges, universities, community-based mental health service providers and higher-education-focused consortia across at least five states, including Washington, Pennsylvania and Mississippi have been trained in the method, and it has been accepted for inclusion in the Substance Abuse and Mental Health Services Administration's National Registry of Evidence-Based Programs and Practices, Cimini says. That means it's been peer-reviewed and is ready to be disseminated.
At the University of Memphis, Murphy's team is further personalizing BASICS by adding a one-hour supplement during which clinicians talk to students about their goals for college and beyond and then show them how their drinking patterns fit in with those aspirations. A student who wants to be a lawyer, for instance, might be given information about a pre-law club as well as the GPA typically needed to get into law school and to earn his desired future salary. The clinician then shows the student a plot based on his responses to an assessment revealing the number of hours per week he typically spends drinking compared with studying or participating in other academic activities. With the graph on hand, the two might then consider potential schedule changes such as dedicating one night a week to law club and another to homework to be more consistent with the student's long-term goals. "Students often [don't] think about their behavior in these sorts of aggregates, and when they're forced to do so," they're motivated to change, Murphy says.
The approach is based on behavioral economics, or the idea that behavior is influenced by availability and cost. In college, where beer is typically cheap and abundant, the framework helps to explain why drinking often gets out of control. But by highlighting appealing alternatives to partying, the approach suggests students will be more likely to steer clear of alcohol's short-lived rewards. "All of that unstructured time, and a lack of awareness of the future benefits of engaging in college or the community, is a lot of what is fueling this binge drinking problem," he says.
The approach appears to be working: In a preliminary study , Murphy's team found that the intervention significantly reduced alcohol problems and heavy drinking among participants. With a new grant from the NIAAA, they're now looking to replicate those findings and track the intervention's long-term effects, on both drinking and college outcomes. "Given that the goals of the intervention are so consistent with the goals of universities, once we can show long-term effects, I'm optimistic that colleges will like it," he says.
Another emerging way to intervene with college drinking targets certain events, rather than people. Twenty-first birthdays are notoriously dangerous: In a 2011 study of 150 students in Psychology of Addictive Behaviors by Fromme and colleagues, participants reported drinking an average of 10.85 drinks on their 21st birthday. Many also experienced blackouts, had unsafe sex and engaged in other risky behaviors.
To keep students safe on that milestone birthday, psychologists are looking at ways to time interventions so that students are reminded to use protective strategies if they plan to celebrate with alcohol. In one study by Neighbors and colleagues, for example, students received one of five BASICS-oriented interventions one week before their 21st birthdays (the interventions varied, with some being Web-based or in person, and some from each group involving a friend). Compared with a control group that received no intervention, the in-person interventions and some of the Web-based ones reduced negative consequences students had on their birthdays. The BASICS interventions that didn't explicitly talk about the risks of 21st birthdays, but rather the risks of drinking in general, reduced both alcohol use and risky behavior, the study found ( Journal of Consulting and Clinical Psychology , 2012).
While the event-specific approach is promising, it's a short-term fix for a larger problem, Neighbors says. "The bigger picture question is: How do we change the culture of drinking on college campuses? It will take more time."
Related Articles
- Parents can influence kids’ drinking in college, research finds

Letters to the Editor
- Send us a letter
- Skip to Content
- Skip to Main Navigation
- Skip to Search

Indiana University Indiana University IU

- Bizarre Medicine
- Health and Medicine Through History
- Conversations in the Abbey, VOL II
- The Field Hospital That Never Was
- Unseen Upton Sinclair
- Conversations in the Abbey
- The Eugenics Movement
- The Progressive Era's Health Reform Movement: A Historical Dictionary (2003)
- Clean Living Movements
- Alcohol and Other Drugs: Self Responsibility
- Controversies in the Addiction Field Sample
- Women: Alcohol and Other Drugs
- More books...
- Original Face Validity and Reliability
- Calculations and Scoring Criteria for the SAQ
- Updated reliability article for SAQ
- Calculations for Health and Lifestyle Questionnaire
- Reliability Article for Health and Lifestyle Questionnaire
- Australian and UK Expanded Version of SAQ
- Reliability Article for Health Concern Questionnaire
- More questionnaires...
- Eugenics, Immigration Restriction and the Birth Control Movements
Why the drinking age should be lowered
- Drinking Patterns and Problems of a National Sample of College Students
- Forbidden Fruit
- Cycles of Social Reform
- Western European Drinking Practices
- Protestants and Catholics
- Drinking games
- Influence of religion and culture
- The Drinking Behaviors - China
- Calculations and Scoring for the Australian and UK SAQ
- Stalking the Determinants of Behavior
- More Works on IUScholarWorks
Alcohol Research and Health History
Why the drinking age should be lowered: an opinion based upon research.
Engs, Ruth C. (1997, 2014). “Why the drinking age should be lowered: An opinion based upon research. Indiana University: Bloomington, IN. Adapted from: IUScholarWorks Repository: http://hdl.handle.net/2022/17594
The legal drinking age should be lowered to about 18 or 19 and young adults allowed to drink in controlled environments such as restaurants, taverns, pubs and official school and university functions. In these situations responsible drinking could be taught through role modeling and educational programs. Mature and sensible drinking behavior would be expected. This opinion is based upon research that I have been involved in for over thirty years concerning college age youth and the history of drinking in the United States and other cultures.
Although the legal purchase age is 21 years of age, a majority of college students under this age consume alcohol but in an irresponsible manner. This is because drinking by these youth is seen as an enticing "forbidden fruit," a "badge of rebellion against authority" and a symbol of "adulthood." As a nation we have tried prohibition legislation twice in the past for controlling irresponsible drinking problems. This was during National Prohibition in the 1920s and state prohibition during the 1850s. These laws were finally repealed because they were unenforceable and because the backlash towards them caused other social problems. Today we are repeating history and making the same mistakes that occurred in the past. Prohibition did not work then and prohibition for young people under the age of 21 is not working now.
The flaunting of the current laws is readily seen among university students. Those under the age of 21 are more likely to be heavy -- sometimes called "binge" -- drinkers (consuming over 5 drinks at least once a week). For example, 22% of all students under 21 compared to 18% over 21 years of age are heavy drinkers. Among drinkers only, 32% of under-age compared to 24% of legal age are heavy drinkers.
Research from the early 1980s until the present has shown a continuous decrease, and then leveling off, in drinking and driving related variables which has parallel the nation's, and also university students, decrease in per capita consumption. However, these declines started in 1980 before the national 1987 law which mandated states to have 21 year old alcohol purchase laws.
The decrease in drinking and driving problems are the result of many factors and not just the rise in purchase age or the decreased per capita consumption. These include: education concerning drunk driving, designated driver programs, increased seat belt and air bag usage, safer automobiles, lower speed limits, free taxi services from drinking establishments, etc.
While there has been a decrease in per capita consumption and motor vehicle crashes, unfortunately, during this same time period there was an INCREASE in other problems related to heavy and irresponsible drinking among college age youth. Most of these reported behaviors showed little change until AFTER the 21 year old law in 1987. For example from 1982 until 1987 about 46% of students reported "vomiting after drinking." This jumped to over 50% after the law change. Significant increase were also found for other variables: "cutting class after drinking" jumped from 9% to almost 12%; "missing class because of hangover" went from 26% to 28%; "getting lower grade because of drinking" rose from 5% to 7%; and "been in a fight after drinking" increased from 12% to 17%. All of these behaviors are indices of irresponsible drinking. This increase in abusive drinking behavior is due to "underground drinking" outside of adult supervision in student rooms, houses, and apartments where same age individuals congregate. The irresponsible behavior is exhibited because of lack of knowledge of responsible drinking behaviors, reactance motivation (rebellion against the law), or student sub-culture norms.
Beginning in the first decade of the 21st century, distilled spirits [hard liquor] began to be the beverage of choice rather than beer among collegians. Previously beer had been the beverage of choice among students. A 2013 study of nursing students, for example, revealed that they consumed an average of 4.3 shots of liquor compared to 2.6 glasses of beer on a weekly basis.
This change in beverage choice along with irresponsible drinking patterns among young collegians has led to increased incidences of alcohol toxicity - in some cases leading to death from alcohol poisoning. However, the percent of students who consume alcohol or are heavy or binge drinkers has been relatively stable for the past 30 years.
Based upon the fact that our current prohibition laws are not working, the need for alternative approaches from the experience of other, and more ancient cultures, who do not have these problems need to be tried. Groups such as Italians, Greeks, Chinese and Jews, who have few drinking related problems, tend to share some common characteristics. Alcohol is neither seen as a poison or a magic potent, there is little or no social pressure to drink, irresponsible behavior is never tolerated, young people learn at home from their parents and from other adults how to handle alcohol in a responsible manner, there is societal consensus on what constitutes responsible drinking. Because the 21 year old drinking age law is not working, and is counterproductive, it behooves us as a nation to change our current prohibition law and to teach responsible drinking techniques for those who chose to consume alcoholic beverages.
Research articles that support this opinion are found in the Indiana University Repository at: https://scholarworks.iu.edu/dspace/handle/2022/17133/browse?type=title
and https://scholarworks.iu.edu/dspace/handle/2022/17130/browse?type=title
Some material here also used in: Engs, Ruth C. "Should the drinking age be lowered to 18 or 19." In Karen Scrivo, "Drinking on Campus," CQ Researcher 8 (March 20,1998):257.
Alcohol Research and Health History resources
(c) Copyright, 1975-2024. Ruth C. Engs, Indiana University, Bloomington, IN 47405

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

Alcohol's Effects on Health
Research-based information on drinking and its impact.
National Institute on Alcohol Abuse and Alcoholism (NIAAA)
Understanding binge drinking, what is binge drinking.
The National Institute on Alcohol Abuse and Alcoholism (NIAAA) defines binge drinking as a pattern of drinking alcohol that brings blood alcohol concentration (BAC) to 0.08%—or 0.08 grams of alcohol per deciliter—or more. This typically happens if a woman has four or more drinks, or a man has five or more drinks, within about 2 hours. 1 Research shows that fewer drinks in the same time frame result in the same BAC in youth: only three drinks for girls and three to five drinks for boys, depending on their age and size. 2

How Common Is Binge Drinking?
According to the 2023 National Survey on Drug Use and Health (NSDUH), about 61.4 million, or 21.7%, of people in the United States ages 12 and older reported binge drinking during the past month. 3,4 Although binge drinking is a concern among all age groups, there are important trends in the following groups.
- Preteens and Teens: Rates of binge drinking among young people have been steadily decreasing in the last decade. Still, according to 2023 data from the Monitoring the Future survey, 2.0% of 8th graders, 5.4% of 10th graders, and 10.2% of 12th graders reported binge drinking in the past 2 weeks. 5
- Young Adults: Rates of binge drinking among people ages 18 to 25 have been decreasing in the past decade, but remain high (28.7% in 2023). 6 According to the 2022 NSDUH, of full-time college students ages 18 to 22, 49.0% drank alcohol and 28.9% engaged in binge drinking in the past month. 6
- Older Adults: According to the 2023 NSDUH, about 12.0% of adults ages 65 and older reported binge drinking in the past month. 4 Binge drinking among this age group is of particular concern because many older adults use medications that can interact with alcohol , have health conditions that can be exacerbated by alcohol, and may be more susceptible to alcohol-related falls and other accidental injuries.
- Women: Studies show that among U.S. women who drink, approximately 1 in 4 have engaged in binge drinking in the last month, averaging about three binge episodes per month and five drinks per binge episode. 7 These trends are concerning because women are at increased risk for health problems related to alcohol misuse .
What Are the Consequences and Health Effects of Binge Drinking?
Although drinking any amount of alcohol can carry certain risks (for information on impairments at lower levels, please see the NIAAA BAC-level infographic ), crossing the binge threshold increases the risk of acute harm, such as blackouts and overdoses . Binge drinking also increases the likelihood of unsafe sexual behavior and the risk of sexually transmitted infections and unintentional pregnancy. These risks are greater at higher peak levels of consumption. Because of the impairments it produces, binge drinking also increases the likelihood of a host of potentially deadly consequences, including falls, burns, drownings, and car crashes.
Alcohol affects virtually all tissues in the body. Data suggest that even one episode of binge drinking can compromise function of the immune system and lead to acute pancreatitis (inflammation of the pancreas) in individuals with underlying pancreatic damage. Over time, alcohol misuse, including repeated episodes of binge drinking, contributes to liver and other chronic diseases as well as increases the risk of several types of cancer, including head and neck, esophageal, liver, breast, and colorectal cancers.
Binge drinking can be deadly. According to the U.S. Centers for Disease Control and Prevention (CDC), approximately 178,000 deaths resulted from excessive alcohol use* annually in the United States between 2020 and 2021. One third of those deaths** were from binge drinking or drinking too much on one occasion. 8
Binge drinking is also costly. Researchers estimated that binge drinking accounted for 77% of the $249 billion (i.e., $191.1 billion) economic cost of alcohol misuse in 2010. 9
How Does Binge Drinking Affect Adolescents?
Brain development, once thought to taper off at the end of childhood, enters a unique phase during the adolescent years. Research indicates that repeated episodes of binge drinking during the teen years can alter the trajectory of adolescent brain development and cause lingering deficits in social, attention, memory, and other cognitive functions. 10
What Is “High-Intensity” Drinking?
High-intensity drinking is defined as alcohol intake at levels twice or more the gender-specific threshold for binge drinking. 11 This dangerous drinking pattern means 8 or more drinks for women and 10 or more drinks for men on one occasion. Research suggests that high-intensity drinking peaks around age 21 and is most common among young adults attending college. 12
This pattern of drinking is of particular concern because it is associated with an even greater risk of severe health and safety consequences. More research is needed to identify interventions that can be used to discourage this pattern of use.
For more information about binge drinking, alcohol use disorder, and available evidence-based treatments, please visit Rethinking Drinking and the NIAAA Alcohol Treatment Navigator .
* CDC defines "excessive alcohol use" as medium or high average daily alcohol consumption. For more information, please see ARDI Methods .
** These deaths include people who were binge drinking, as well as people whose deaths were caused by someone else's binge drinking, such as in traffic fatalities.
1 NIAAA. [Internet.] Defining binge drinking. What colleges need to know now. Bethesda (MD): National Institutes of Health; 2007 Nov. [cited 2023 Feb 20]. Available from: https://www.collegedrinkingprevention.gov/sites/cdp/files/documents/1College_Bulletin-508_361C4E.pdf
2 Chung T, Creswell KG, Bachrach R, Clark DB, Martin CS. Adolescent binge drinking: developmental context and opportunities for prevention. Alcohol Res. 2018;39(1):5-15. PubMed PMID: 30557142
3 SAMHSA. Center for Behavioral Health Statistics and Quality. 2023 National Survey on Drug Use and Health. Table 2.9A—Alcohol, binge alcohol, and heavy alcohol use in past month: among people aged 12 or older; by detailed age category, numbers in thousands, 2022 and 2023. [cited 2024 Aug 14] Available from: https://www.samhsa.gov/data/report/2023-nsduh-detailed-tables
4 SAMHSA. Center for Behavioral Health Statistics and Quality. 2023 National Survey on Drug Use and Health. Table 2.9B—Alcohol, binge alcohol, and heavy alcohol use in past month: among people aged 12 or older; by detailed age category, percentages, 2022 and 2023. [cited 2024 Aug 14] Available from: https://www.samhsa.gov/data/report/2023-nsduh-detailed-tables
5 Miech RA, Johnston LD, Patrick ME, O'Malley PM. (2024). Monitoring the Future National Survey Results on Drug Use, 1975—2023: Overview and Detailed Results for Secondary School Students. Table D-33: Alcohol: Trends in use over various prevalence periods in grades 8, 10, and 12. [cited 2024 Aug 23]. Available from: https://monitoringthefuture.org/wp-content/uploads/2024/01/mtfoverview2024.pdf
6 Past-month alcohol use: consuming a drink of a beverage containing alcohol (a can or bottle of beer, a glass of wine or a wine cooler, a shot of distilled spirits, or a mixed drink with distilled spirits in it), not counting a sip or two from a drink in the past 30 days. Past-month binge drinking: consuming five or more drinks on the same occasion for males or four or more drinks on the same occasion for females on at least 1 day in the past 30 days. The population prevalence estimate (%) is weighted by the person-level analysis weight and derived from the Center for Behavioral Health Statistics and Quality 2022 National Survey on Drug Use and Health (NSDUH-2022-DS0001) public-use file. [cited 2024 Jan 12]. Available from: https://www.datafiles.samhsa.gov/dataset/national-survey-drug-use-and-health-2022-nsduh-2022-ds0001
7 Kanny D, Naimi TS, Liu Y, Lu H, Brewer RD. Annual total binge drinks consumed by U.S. adults, 2015. Am J Prev Med. 2018;54(4):486-96. PubMed PMID: 29555021
8 CDC. [Internet.] Facts about U.S. deaths from excessive alcohol use. [cited 2024 Aug 30]. Available from: https://www.cdc.gov/alcohol/facts-stats/index.html
9 Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD. 2010 national and state costs of excessive alcohol consumption. Am J Prev Med. 2015;49(5):e73-e79. PubMed PMID: 26477807
10 Jones SA, Lueras JM, Nagel BJ. Effects of binge drinking on the developing brain. Alcohol Res. 2018;39(1):87–96. PubMed PMID: 30557151
11 Hingson RW, Zha W, White AM. Drinking beyond the binge threshold: predictors, consequences, and changes in the U.S. Am J Prev Med. 2017;52(6)717–27. PubMed PMID: 28526355
12 Patrick ME, Azar B. High-intensity drinking. Alcohol Res. 2018;39(1):49–55. PubMed PMID: 30557148
niaaa.nih.gov
An official website of the National Institutes of Health and the National Institute on Alcohol Abuse and Alcoholism
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Binge Drinking in Young Adults: Data, Definitions, and Determinants
Kelly e. courtney.
Department of Psychology, San Diego State University, La Jolla, California
John Polich
Molecular and Integrative Neurosciences Department, The Scripps Research Institute, La Jolla, California
Binge drinking is an increasingly important topic in alcohol research, but the field lacks empirical cohesion and definitional precision. The present review summarizes findings and viewpoints from the scientific binge-drinking literature. Epidemiological studies quantify the seriousness of alcohol-related problems arising from binge drinking, with a growing incidence reported in college-age men over the last 2 years. Experimental studies have found neurocognitive deficits for frontal lobe processing and working memory operations in binge-drinking compared with nonbinge alcohol drinkers. The findings are organized with the goals of providing a useful binge-drinking definition in the context of the empirical results. Theoretical implications are discussed on how binge drinking may alter neurophysiological and neurocognitive function.
Alcohol consumption in humans is the third leading preventable cause of death in the United States ( McGinnis & Foege, 1993 ). A common abuse pattern called binge drinking contributes to a substantial portion of alcohol-related deaths ( Chikritzhs, Jonas, Stockwell, Heale, & Dietze, 2001 ). This type of drinking also is associated with alcohol poisoning, unintentional injuries, suicide, hypertension, pancreatitis, sexually transmitted diseases, and meningitis, among other disorders. As binge drinking is relatively common, it underlies many negative social costs, including interpersonal violence, drunk driving, and lost economic productivity, as reported by the National Institute on Alcohol Abuse and Alcoholism ( NIAAA, 2000 ). These statistics have attracted increased attention from a variety of perspectives.
The term “binge” originated as a clinical description of alcoholics and was defined by periods of heavy drinking followed by abstinence ( Tomsovic, 1974 ). The word is distinct from the expression “binge drinking” that, since its conception, has engendered a wide array of definitional elements. This definitional difficulty originates from two different but related uses of the phrase: (1) epidemiological studies that emphasize isolated excessive drinking episodes, and (2) experimental studies that evaluate behavioral drinking patterns ( Lange & Voas, 2000a ). The present review was undertaken to bridge these approaches and to provide a comprehensive, integrative, and useful portrait of the binge-drinking literature with a focus on young adult humans. We obtained studies through literature searches using “binge drinking,” “alcohol binging,” and “college drinking.” Ancillary terms, such as light or social drinking and alcohol dependence, were included when they occurred within the binge framework ( Boyd, McCabe, & Morales, 2005 ). The goals were to characterize the primary data and definitional attributes of binge drinking as delineated by current scientific findings.
Table 1 summarizes the binge-drinking studies identified. Although the conceptual and empirical views of an operational definition have been slow to coalesce, technical agreement about binge drinking has evolved appreciably over the last 10 years. Specific reports are used to illustrate how the definition, its rationale, and utility have developed. The approach considers both quantity and frequency of consumption as defining characteristics of binge drinking. The review is organized into three sections: (1) Issues underlying the concept of binge drinking are outlined; (2) the relationship of alcohol consumption to binge drinking is highlighted; (3) binge drinking and its cognitive, physiological, and withdrawal effects are examined, with the influence of alcoholism, family history for alcoholism, and other determinants sketched. In the Discussion section, we review the implications of the findings and suggest future research directions.
| Author (year) | Binge definition | Population | Age (years) | Method | Conclusion | |
|---|---|---|---|---|---|---|
| 5/4+ drinks past 2 weeks | U.S. college students | F = 10,203 M = 7,389 | N/A | Survey (CAS) | 44% binge drinkers, 19% frequent binge drinkers, 47% frequent binge drinkers who had 5+ drinking-related problems. | |
| Developed the 5/4 measure | U.S. college students | = 12,243 | N/A | Survey (CAS) | Women who drink 4 drinks in a row have same likelihood of experiencing drinking-related problems as men who drink 5 drinks in a row. | |
| 5/4+ drinks past 2 weeks | U.S. college students | F= 10,203 M = 7,389 | N/A | Survey (CAS) | Prior high school binging; gender and race significant correlates; predictors = fraternity or sorority, party-centered life, and risky behaviors. | |
| 10+ drinks <2 days/week, irregular basis | Referred from alcohol-related brain injury support groups | F= 18 M = 82 | 25–68 | Neuropsychological tests | Semantic organizational ability poorer in constant drinkers versus bingers; executive performances on tasks are similar for both groups. | |
| Being “drunk or very high from alcohol” in past 90 days | U.S. high schools | F= 159 M = 111 | 16–18 | Scenarios describing drinking situations, questionnaires | ||
| 5/4 at one time | U.S. college students | F = 72 M = 40 | 17–43 | Questionnaire | 38.6% of those that binged during the past 3 months would not have been identified as bingers using a 2-week time period; age, church attendance, alcohol-related consequences, and age of first intoxication were the same for bingers identified from both time periods. | |
| 5+ drinks/occasion (all subjects met – diagnostic criteria for alcohol dependence) | General community | F = 99 M = 70 | M = 30.1 | Interviews—random telephone | Positive relationship between family history and chronicity (stable relationship between familial/genetic background and alcohol dependence). | |
| 0.08 + BAC | U.S. drinkers in Tijuana | F = 512 M = 547 | 18–33 | Survey | 5/4 measure too low for 0.08 BAC; should use 6/5 measure. | |
| 5/4+ drinks past 2 weeks | N/A | N/A | N/A | Discussion of binge definition | 5/4 measure extensively used and chosen because it provides a simple way to assess a drinking style that threatens the public's health. | |
| 5/4+ drinks per occasion | General community | F = 8 M = 26 | 24–38 | Questionnaires: alcohol/drug experimental: BAC, heart rate, Cortisol levels | Young adult binge drinkers show a biphasic BAC alcohol response compared with light drinkers. | |
| Binge/episodic: 5/4+ drinks per occasion Heavy episodic drinker = within past 2 weeks (occasional = 1/2 times, within 2 weeks; frequent = 3+ times within 2 weeks) | U.S. college students (CAS) | F = 8,610 M = 5,505 | N/A (50.2% <21) | Survey | 31% of students endorsed criteria for an alcohol abuse diagnosis and 6% for a dependence diagnosis in past 12 months. Heavy episodic drinkers more likely to have an alcohol disorder. Frequent heavy episodic drinkers had 13 times greater odds for abuse and 19 times greater odds for dependence. | |
| Binge Drinking Score: Drinks per hour, times drunk within last 6 months, percentage of being drunk when drinking (4 × Item − 10 + 1 × Item − 11 + 0.2 × Item − 12 = drinking pattern) | University of Sussex students | F = 49 M = 47 | 18–34 | Neuropsychological tests | Acute alcohol consumption impairs executive-type cognitive functions, and binge drinking may be associated with impaired cognitive functioning in a working memory and pattern recognition task. | |
| Binge drinking score | U.S. college students | F = 41 M = 14 | 18–28 | Alcohol Use Questionnaire (AUQ), diary | High drinkers tended to underestimate their drinking behavior, whereas lower drinkers tended to overestimate. Found that relationship between binge scores, beverage specificity, and alcohol consumption supports idea that binge drinking criteria should be based on patterns of drinking rather than alcohol consumption. | |
| 5/4 per drinking episode | General community | F = 32 M = 44 | 18–32 | Questionnaires | Lower self-efficacy and greater positive alcohol expectancies predicted greater numbers of follow-up binge episodes. Greater positive alcohol expectancies predicted greater follow-up alcohol consumption. | |
| 5+ drinks on 1 occasion | General community | = 724,479 F = 19% of bingers M = 81% of bingers | 18+ | Survey (BRFSS—random telephone) | Per capita binge-drinking episodes have increased since 1995; men account for 81% of binge drinking episodes, and 69% of binge-drinking episodes occurred among those 26 years of age or older. | |
| 5/4+ drinks in a row | Caucasian undergraduate college students | F = 147 M = 57 | M = 19 | Survey, genetic analysis | Students who were homozygous for the 5-HTTLPR short allele were more likely to engage in binge drinking behavior, drank more alcohol per occasion, and reported drinking to get drunk more often, as compared with homozygous or heterozygous for the long allele. | |
| 5/4+ consecutive drinks per occasion 1 + times in past 2 weeks | First-year college students | F = 1,263 M = 631 | ≤19, first-year | Survey (CAS data) | Students who reported that they were exposed to “wet” environments (prevalent and cheap alcohol availability) were more likely to engage in binge drinking than peers without exposure. | |
| 5+ drinks on 1 occasion | General community (Dutch) | F = 24 M = 24 | M =21.9 | Neuropsychological tests | Memory retrieval processes significantly impaired during an alcohol hangover after binge drinking. | |
| 5+ drinks in a row “Heavy episodic drinking” | U.S. college students | F = 885 M = 724 | 18–61 | Survey, interviews (telephone) | Having many people, available illicit drugs, “BYOB” events, playing of drinking games predicts heavy episodic drinking; students actively seek out environments with characteristics that facilitate heavy drinking. | |
| Binge Drinking Score | King's College students | F = 12 M = 15 | 18–23 | Neuropsychological tests | Binge drinkers compared with nondrinkers have lower trait anxiety and depression, as well as poorer performance for sustained attention, episodic memory, and planning ability. | |
| 0.08 + BAC (approximately = 5/4+ drinks in 2 hr) | N/A | N/A | N/A | Definition | Drink = a half ounce of alcohol. | |
| 6/4+ drinks per drinking period in past 2 weeks | N/A | N/A | N/A | Theoretical article | Proposes a cognitive model for binge drinking that uses alcohol expectancies and drinking refusal self-efficacy to explain the acquisition and maintenance of binge drinking. Suggests that the combinations of alcohol and drinking refusal self-efficacy can explain the four drinking styles (social, binge, heavy, and alcoholic). | |
| 100/80+ drinks per month (<21 days) in past 3 years (about = 5/4+ drinks in a row) | General community | F = 28 M = 70 | M = 41 | MRI and MRSI, neurocognitive tests | Binge drinking modulates brain metabolite abnormalities. | |
| 5+ drinks on 1 occasion in past month (frequent = 3+ days, infrequent = 1–2 days) | General community | F = 44,995 M = 54,788 | Frequent bingers: M = 34.6; infreq bingers: M = 35.6; nonbingers: M = 45.7 | Survey (BRFSS—random telephone), HRQOL | In 2001, 11% of current drinkers were frequent bingers (3+ in past month), and 14% were infrequent (1–2 in past month). Frequent bingers more likely than nonbingers to have ≥ 14 unhealthy days in past month (unhealthy = stress depression and emotional problems). | |
| 5+ drinks on 1 occasion 1 + times in past 30 days (NHSDA definition) | Adolescents and college students | N/A | N/A | Review | Underage alcohol use is associated with brain damage and neurocognitive deficits. | |
| N/A | College students | N/A | N/A | Review | Provides a review on the biology, identity, cognition, affiliation, and achievement of college student alcohol use. | |
| [original authors of Binge Drinking Score] | Binge Drinking Score | General community (England) | F = 47 M = 50 | 18–30 | Neuropsychological tests | Cognitive performance and mood differences between binge and nonbinge drinkers: Movement time on 4 and 8 patterns, and MTS choice time on 8 pattern condition faster in bingers; female bingers worse on SWM and vigilance task than female nonbingers. |
| 5+ drinks in a row on 1 + of the 30 days preceding survey | U.S. high schools | F = 6,889 M =7,028 | Grades 9–12 | Survey (Youth Risk Behavior Surveillance. 2005) | Episodic heavy drinking higher among male (27.5%) than female (23.5%) students. | |
| SAMHSA (2005) | 5+ drinks on 1 occasion 1 + times in past 30 days | General community | = 68,308 | 12 + | Survey (National Household Survey on Drug Abuse) | Other researchers derive actual findings from survey outcomes. |
| 5/4+ drinks in past 12 months | U.S. college students | F = 2,304 M = 2,276 | M = 19.9 | Web survey | Past month time-frame lead to higher prevalence estimate than 2-week time-frame and was positively associated with negative consequences. | |
| 5/4+ drinks in a row past 2 weeks | U.S. college students | N/A | N/A | Review binge cutoff points | 5/4 measure valuable for assessing alcohol related harms in a college population. | |
| 5+ drinks per occasion | Southwest California Indians | F = 73 M = 52 | M = 19.9 | ERP (facial discrimination task) | Adolescent exposure (alcohol and drugs) associated with latency decrease in P350 and amplitude decrease in P450. | |
| 5+ on one occasion 2+ times in the past 30 days | U.S. college students | F = 25 M = 25 | M = 20 | Iowa Gambling Task (IGT) questionnaires | Stable high binge drinking group made less advantageous choices on the IGT than the low binge drinking group. Impulsivity was not related to decision-making performance. | |
| 5/4+ drinks in a row | U.S. college students | F = 182 M = 355 | M = 18.6 | Questionnaires | Nearly 1/3 of those classified as nonbinge drinkers in the second 2 weeks of the month were classified as either binge drinkers or frequent binge drinkers in the first 2 weeks of the month. | |
| Standard binge = 5/4+ drinks per occasion; Heavy binge = 7/6+ drinks per occasion | U.S. college students | F = 184 M = 172 | M = 19 | Questionnaires | Binge drinkers differed from heavy binge drinkers on actual drunkenness (measured by estimated blood alcohol levels); only the heavy binge drinkers differed from the nonbinge drinkers on measures of total alcohol consequences and drinking frequency. |
Note. F = female; M = male; N/A = not applicable; CAS = College Alcohol Study; BAC = blood alcohol concentration; BRFSS = Behavioral Risk Factor Surveillance System; BYOB = “bring your own booze”; NIAAA = National Institute on Alcohol Abuse and Alcoholism; MRI = magnetic resonance imaging; MRSI = magnetic resonance spectroscopic imaging; HRQOL = health-related quality of life; NHSDA = National Household Survey on Drug Abuse; MTS = Matching to Sample Visual Search task; SWM = Spatial Working Memory task; SAMHSA = Substance Abuse and Mental Health Services Administration; ERP = event-related potential.
Definitional Background
An initial view defined binge drinking as at least five alcoholic drinks consumed during the same session ( Cahalan, Cisin, & Crossley, 1969 ). However, the comprehensive College Alcohol Study (CAS) conducted by the Harvard School of Public Health characterized binge drinking as five drinks for men and four drinks for women on a single occasion within the past 2 weeks ( Wechsler, Davenport, Dowdall, Moeykens, & Castillo, 1994 ). The adjustment to the four-drink cutoff for women was based on their lower rate of gastric metabolism for alcohol, which leads to higher blood alcohol levels compared with men for the same quantity ( Wechsler, Dowdall, Davenport, & Rimm, 1995 ). The 5/4 definition is consistent with findings that after consumption of this amount or more, individuals are at greater risk for exhibiting serious alcohol-related problems (e.g., vandalism, fights, injuries, drunk driving, trouble with police, etc.) and subsequent negative health, social, economic, or legal consequences (Wechsler, 2000).
Despite the intended practicality of the CAS and other large scale survey definitions, characterizing binge drinking using only a “single occasion” within a specified time-frame may conflate the estimates of binge drinkers as defined by a pattern of behavior ( Naimi et al., 2003 ; Substance Abuse and Mental Health Services Administration [SAMHSA], 2007 ; Wechsler et al., 1994 ), as both drinking quantity and frequency have been shown to be important indictors of risky drinking in college students ( Presley & Pimentel, 2006 ). Additional issues include how a single “drink” is defined, consumption amount, and alcohol tolerance contribute to individual inebriation levels ( Jaccard & Turrisi, 1987 ).
One attempt to quantify behavioral drinking employed blood alcohol concentration (BAC) level, such that a 0.08 gram percent—now the legal intoxication level in all 50 states ( Alcohol Policy Information System, 2007 )—for a given occasion indicated binge-drinking patterns ( Lange & Voas, 2000b ). Another approach developed a Binge-Drinking Score from three questions of the Alcohol Use Questionnaire ( Mehrabian & Russell, 1978 ; Townshend & Duka, 2002 ). This method used quantifiable assessments of drinks per hour, times drunk within the last 6 months, and percentage of time being intoxicated when drinking to calculate a summary score unrelated to the weekly consumption of alcohol ( Townshend & Duka, 2005 ).
A standardized conceptual definition of binge drinking was proposed by the NIAAA in 2004:
A “binge” is a pattern of drinking alcohol that brings BAC to 0.08 gram percent or above. For the typical adult, this pattern corresponds to consuming five or more drinks (male), or four or more drinks (female), in about two hours. (p. 3)
A standard drink equals 0.5 oz of alcohol as is found in one 12-oz beer, one 5-oz glass of wine, or one 1.5-oz shot of distilled spirits ( NIAAA, 2004 ). This definition of binge drinking is similar to many used in epidemiological studies, which employ quantity (BAC), consumption amounts, and episode duration. The definition does not specify, however, the time period or number of binging occurrences that would describe a long-term binge-drinking practice. Thus, NIAAA's definition characterizes single binge episodes but does not capture the consumption pattern associated with serious health and social consequences.
The inclusion of a past time-frame to quantify frequency of binging episodes is necessary to differentiate “binge drinking” from “alcoholism” or “alcohol dependence.” This temporal aspect of a binge-drinking pattern has been variably defined as the past week ( Kokavec & Crowe, 1999 ), past 2 weeks ( Wechsler et al., 1994 ), past 30 days/month ( Okoro et al., 2004 ; SAMHSA, 2007 ; Zeigler et al., 2005 ), past 6 months ( Hartley, Elsabagh, & File, 2004 ; Townshend & Duka, 2002 , 2005 ; Weissenborn & Duka, 2003 ), and past year ( Cranford, McCabe, & Boyd, 2006 ). These different time-frames emphasize various aspects of binge-drinking patterns, but their use inhibits direct comparison among findings.
The most informative time-frame appears to be within the past 6 months, as it is an optimal period to link alcohol consumption and alcohol-related problems ( Hartley et al., 2004 ; Townshend & Duka, 2002 , 2005 ; Weissenborn & Duka, 2003 ). Longitudinal studies of binge drinking have established that college students inconsistently report heavy episodic drinking across time ( Schulenberg, O'Malley, Bachman, Wadsworth, & Johnston, 1996 ; Weingardt et al., 1998 ), so that a 2-week time-frame would underestimate binging prevalence ( Vik, Tate, & Carrello, 2000 ). A recent study found that nearly one third of those classified as nonbinge drinkers (<5/4 drinks) during a 2-week time period in the middle of the month were classified as either binge drinkers (≥5/4 drinks, 1 or 2 times during the past 2 weeks) or frequent binge drinkers (≥5/4 drinks, ≥3 times in past 2 weeks) during the first 2 weeks of the month ( LaBrie, Pedersen, & Tawalbeh, 2007 ). Use of a 2-week time period, therefore, would yield approximately 30% of heavy binge drinkers being excluded. A past 6 months time-frame for college samples captures the vacation time of the academic calendar during which students would be more apt to binge drink. Although longer time frames have yet to be analyzed, the ability to recall consumption amounts and frequencies accurately (e.g., recall bias) would seem to diminish with extended time frames. The goal in selecting an optimal time frame associated with a binge-drinking pattern is to optimize the accuracy of self-reported drinking amounts, while also capturing an accurate representation of this problematic drinking pattern. Further, employing a multiple binging occurrences evaluation strengthens the definition as these attributes together integrate the quantifiable dimensions of binge drinking.
Epidemiology
The age of onset of regular (> once a month) drinking has been reported to be “15.2 ± 1.2 years old ( M ± SD ) for high-risk children and 16.5 ± 1.2 years old for low-risk children” on the basis of a sample of 125 children ( Hill, Shen, Lowers, & Locke, 2000 , p. 269). Of the total 10.8 million underage Americans (12–20 years) who reported consuming alcohol in the past 30 days, 7.2 million (or 19%) were binge drinkers (≥5 drinks on the same occasion on ≥1 day in past 30 days) as defined by National Survey on Drug Use and Health ( SAMHSA, 2007 ). Early onset of binge drinking or exposure to binging has been linked to the increased risk of binging in adulthood ( Wechsler, Dowdall, Davenport, & Castillo, 1995 ; Weitzman, Nelson, & Wechsler, 2003 ). Other factors that predict binging include the following: never being married, having a grade point average of B or less, and placing little importance on religion.
The CAS study found that for a sample of 140 colleges nationwide, 44% of the responding students were binge (≥5/4 successive drinks) drinkers ( Wechsler et al., 1994 ). The Behavioral Risk Factor Surveillance System (BRFSS) study assessed adults who were 18 years of age or greater through a random-digit telephone survey across the United States between 1993 and 2001 ( Naimi et al., 2003 ). The number of binge episodes (≥5 alcoholic beverages in one sitting) among adults in the United States increased from about 1.2 billion to 1.5 billion. The younger adults in this sample (18–25 years) evinced the highest rate of binge-drinking episodes in the year 2001, whereas individuals older than 55 years had the lowest rate of binge-drinking episodes ( Naimi et al., 2003 ). Differences in the prevalence estimates (CAS vs. BRFSS) may be due to different populations, with the CAS targeting college students and the BRFSS targeting the general community.
Most epidemiological reports indicate that men account for the majority of binge drinkers ( Cranford et al., 2006 ; Wechsler et al., 1994 ; Wechsler, Dowdall, Davenport, & Castillo, 1995 ). The CAS study found that approximately 50% of the male and 39% of the female students were binge drinkers, with the BRFSS study concluding that men accounted for 81% of all binge-drinking episodes ( Naimi et al., 2003 ). Furthermore, bingers in the BRFSS study were less likely to report any college education compared with nonbingers, although the opposite outcome also has been reported ( Dawson, Grant, Stinson, & Chou, 2004 ; Slutske, 2005 ).
Racial differences were reported. Being White accounted for 78% of all binge-drinking episodes, and Hispanics demonstrated the highest rate of binge-drinking episodes per person for most of the years examined. African Americans constituted the lowest binge-drinking racial group, with fewer than five episodes per person per year ( Naimi et al., 2003 ). Another large scale survey ( N = 4,580) found a 33.2% prevalence estimate for binging (≥5/4 drinks in a row during past 2 weeks) for Asians compared with a 60.7% prevalence estimate for Whites ( Cranford et al., 2006 ). The high frequency of a “flushing response” after alcohol ingestion has been theorized to account for the lower binging rates in Asians. The aldehyde dehydrogenase gene (ALDH2, Chromosome 12) that is prevalent in Asian populations fosters severe and predominately negative reactions to a moderate dose of alcohol compared with a heterozygous or individual without the allele ( Cook et al., 2005 ).
Alcohol Consumption
Alcohol's effect on individuals stems from a variety of cognitive, biological, and social factors. The propensity to binge drink may arise from a combination of these factors, which could contribute to the underlying “cause” of binge drinking. Studies of these factors typically employ drinking definitions that are specialized for the particular variable or measure used, so that result comparisons need to be made from this perspective. However, these variables taken in the context of their roles as mediators and moderators of alcohol consumption are potentially important indices of future binge drinking and are reviewed here to provide appropriate background for their effects.
Alcohol Expectancies
Alcohol impairs the functioning of a variety of domains, including memory, judgment, and behavior ( Nelson et al., 1998 ; Sayette, 1999 ). It diminishes eye movements ( Blekher et al., 2002 ; Holdstock & de Wit, 1999 ; Moser, Heide, & Kömpf, 1998 ), short-term memory ( Chait & Perry, 1994 ; Heishman, Arasteh, & Stitzer, 1997 ; Mattila et al., 1996 ), and motor performance ( Fogarty & Vogel-Sprott, 2002 ). These direct influences of alcohol consumption, however, vary in magnitude as a function of amount ingested and individual differences in alcohol expectancies. A study of 302 undergraduates found that mood was affected by alcohol intake: Men more often reported social-situational enhancements (e.g., meeting people), whereas women often reported physical (e.g., falling asleep) effects ( Goldstein, Wall, McKee, & Hinson, 2004 ). Alcohol-related memories can account for as much as 50% of the variance in predicting concurrent and prospective drinking ( Wiers et al., 2002 ), and expectances can predict as well as demographic variables, such as social and problem drinking ( Christiansen & Goldman, 1983 ).
Expectancy effects can be manipulated: Drinkers instructed to “try and stay sober” demonstrated superior hand coordination and recall memory performance compared with those not so motivated ( Young & Pihl, 1980 ). Lower numbers of positive alcohol expectancies and reduced consumption have been linked to fewer binge-drinking episodes, whereas negative expectancies were not ( Blume, Schmaling, & Marlatt, 2003 ). Alcohol expectancies and drinking refusal self-efficacy have been proposed to be significant predictors of drinking styles. Binge drinkers (≥6/4 drinks per drinking period) were characterized as either having positive (are able to refuse drinks easily) or negative (unable to stop drinking) drinking refusal self-efficacy. A model derived from these observations “predicts that social and binge drinkers can be discriminated on the basis of their alcohol expectancies, while binge drinkers and alcoholics can be discriminated on the basis of drinking refusal self-efficacy” ( Oei & Morawska, 2004 , p. 173). Thus, beliefs about alcohol effects appear to contribute to the experience of drinking.
Perception of Drunkenness
Inebriation is another important factor related to binge drinking, and it is often reported as the basis for binging ( Wechsler et al., 1994 ). However, alcohol drinkers misbelieve that standard mixed drinks are more potent than standard servings of wine or beer. These individuals also believe that each additional drink they consumed had a decreasing impact on BAC ( Jaccard & Turrisi, 1987 ). Sober adolescents were asked to estimate their perceived level of simulated drunkenness as quantified by whether their BAC was under or over the legal limit while they were exposed to external cues that systematically described drinking scenarios ( Turrisi & Wiersma, 1999 ). The young people underestimated their “perceived” level of inebriation during 19% of the experimental scenarios, suggesting that their judgment was affected by the cues.
Induced public self-awareness (stimulated by exposure to mirrors and a camera) was hypothesized to increase salience of the situational behavioral standard (i.e., sober comportment), which increased motivation toward effortful performance. Shorter response time was obtained for the self-aware compared with the control group on a task that required the participant to identify correct and misspelled words ( Ross & Pihl, 1988 ). This expectancy effect also was observed for at-risk college drinkers trained to reduce consumption by demonstrating that the students experienced enhanced mood and conviviality when they were induced to think they were consuming alcohol but were not ( Fromme, Marlatt, Baer, & Kivlahan, 1994 ). As greater positive expectancies have been associated with binge drinking, expectancy differences appear to be a strong influence on alcohol's individual effects ( Blume et al., 2003 ).
Tolerance to Alcohol
Individual responsivity or “tolerance” to alcohol also is important and has been assessed by the BAC curve changes with consumption ( Fillmore & Vogel-Sprott, 1998 ). The rising limb theory supposes that heavy drinkers are more sensitive than light drinkers to the subjective positive euphorigenic effects during the early portion of the BAC curve but less sensitive to the sedative-like effects during both the rising and declining phases ( Holdstock, King, & de Wit, 2000 ). Young adult heavy binge drinkers (≥5/4 drinks on one occasion at least once a week) were found to produce this biphasic response on a battery of behavioral scales. An initial pattern of positive reinforcement and absence of negative effects was obtained for the binge compared with nonbinge drinkers (<5/4 drinks per occasion), who did not show a biphasic alcohol response and reported heightened sedation throughout both limbs of the BAC curve ( King, Houle, de Wit, Holdstock, & Schuster, 2002 ). Although the biphasic response may have been produced by the binge pattern of consumption, the authors speculated that the differential sensitivity between binge and nonbinge drinkers may have contributed to the enhanced risk for the development of alcohol-use disorders and the acquisition of binge-drinking patterns.
Social Issues
Drinking in a group leads to the experience of greater euphoria than drinking the same quantity alone ( Pliner & Cappell, 1974 ), and drinking in a social setting facilitates more consumption than solitary drinking ( Storm & Cutler, 1981 ). A survey of 409 college students found that a drinking event with many people intoxicated and having school friends present were factors predictive of binge drinking with five or more drinks ( Clapp & Shillington, 2001 ). Students often seek out environments that facilitate binge drinking ( Clapp et al., 2003 ; Lange & Voas, 2000b ). Indeed, peer relationships can be a risk factor for increased alcohol consumption, as collegiate living arrangements—especially fraternities and sororities—are a significant correlate of binge drinking. Other factors include living with a roommate, stressing the importance of parties, and having five or more close student friends ( Wechsler, Dowdall, Davenport, & Castillo, 1995 ).
Binge drinking can affect quality of life in terms of general health. After adjustment for age, frequent binge drinkers (≥5 drinks on one occasion > 3 times in last 30 days) compared with infrequent binge drinkers (≥5 drinks on one occasion < 3 times in the last 30 days) were more likely than nonbinge drinkers to report fair or poor health and experience more sick days. These findings appear to reflect the generally negative consequences of alcohol abuse but at an earlier stage in poor health development ( Okoro et al., 2004 ).
In contrast, the benefits of light and moderate alcohol consumption have been well documented for stress reduction, mood enhancement, reduced depression symptoms, improved functioning in the elderly ( Baum-Baicker, 1985 ; Pernanen, 1991 ), as well as for protection against coronary artery disease ( Sacco et al., 1999 ). These issues often are reported as reasons for consuming alcohol. Only when the perceived drinking effects are detached from personal experience are harmful effects of drinking cited as “objective” assessments ( Peele & Brodsky, 2000 ). The term “moderate” drinking, therefore, should not be confused with “binge drinking,” as the latter implies irregular intake and withdrawal from large quantities of alcohol and often leads to different outcomes than the positive ones associated with moderate drinking.
Binge Drinking
The current binge-drinking literature varies widely on the nature of the individual studies and definitions used to categorize alcohol consumption. Interpreting the results of these studies, therefore, requires a perspective that includes comparative awareness of sample characteristics, binge-drinking definition, and the control/nonbinge-drinking group inclusion criteria. Important too is to maintain the distinction between human and animal studies, as the former are typically much less specific than the later with respect to the neurophysiological underpinnings of binge-drinking effects. However, an overview of the general findings helps provide a fundamental grounding in what is known about binge-drinking outcomes at different levels of effect.
Cognitive Effects
Binge-drinking studies that measure cognitive function have found frontal lobe and working memory deficits, although an empirical definition of binging has not been used consistently. Heavy social drinkers, defined to include those who engaged in binge-drinking episodes, demonstrated delayed auditory and verbal memory deficits that were related to task difficulty. These deficits were not found for the light social drinkers. The findings implied that “frequent intake of large amounts of alcohol in any one sitting (i.e., ‘binge’ drinking) may place individuals at an increased risk for suffering alcohol-related cognitive impairment” ( Nichols & Martin, 1997 , p. 455). However, the conflation of participant drinking levels with descriptive labels colors statements about binge-drinking effects, thereby making comparisons unclear.
In Table 2 , we summarize neurocognitive studies of binge-drinking studies using standard neuropsychological tests. The Binge-Drinking Score method was employed in several of these to define research participant drinking groups ( Townshend & Duka, 2005 ). Binge drinkers compared with nonalcohol drinkers evinced cognitive impairments in the Paced Auditory Serial Addition Test, executive planning function, and episodic memory tasks—findings similar to frontal function deficits found in Korsakoff alcoholics ( Hartley et al., 2004 ). Another report found that binge drinkers relative to nonbinging drinkers produced errors in a spatial working memory and pattern recognition tasks ( Weissenborn & Duka, 2003 ). Furthermore, female compared with male binge drinkers were more impaired on these paradigms and unable to inhibit their response to an alerting stimulus in a vigilance task. Thus, binge drinking may be associated with deficits in frontal inhibitory control ( Townshend & Duka, 2005 ).
| Sample size | |||||
|---|---|---|---|---|---|
| Study (year) | Study characteristics | F | M | Tasks | Outcome (C = Control, B = Binge) |
| Binge: Consume >10 drinks irregularly (<2 days per week) | 12 | 38 | WMS-R Logical Memory | C > B | |
| Nonbinge: Consume >10 alcoholic drinks/day | 6 | 44 | WMS-R Visual Reproduction | C = B | |
| Conditions: All subjects diagnosed alcohol dependent, detoxified, gender/family history matched, subjects abstained from alcohol >2 weeks prior, no smoking data on subjects | Rey Complex Figure | C = B | |||
| Rey Auditory Verbal Learning Test | |||||
| Trail Making Test | C = B | ||||
| C = B | |||||
| Binge: 0.8 g/kg of 90% volume/volume alcohol in 30 min | 24 | 24 | CANTAB–Tower of London | C > B | |
| CANTAB–Spatial Recognition Task | C > B | ||||
| Nonbinge: placebo group | 25 | 22 | CANTAB–Spatial Working Memory Task | C = B | |
| Conditions: Alcohol challenge, no alcohol abuse, subjects abstained from alcohol for >12 hr prior, smoking data unreported, gender taken into account | CANTAB–Pattern Recognition Task | C = B | |||
| Binge: Moderate alcohol drinkers 8–9 standard drinks (1.4 g/kg body weight) in 30 min | Not reported | Word Learning Test–Learning | C > B | ||
| Word Learning Test–Delayed Recall | C > B | ||||
| Nonbinge: Moderate alcohol drinkers in placebo | Word Learning Test–Immediate Recall | C = B | |||
| Word Learning Test–Recognition Score | C = B | ||||
| Conditions: Alcohol challenge, no alcohol dependency, gender taken into account, <10 cigarettes per day allowed, no alcohol recency data | Word Learning Test–Recognition Time | C = B | |||
| Mackworth Clock Test | C = B | ||||
| Binge: Binge Drinking Score > cutoff, 10 units (8 g alcohol) per occasion, ≥5 M (≥4 F) per occasion | 5 | 9 | Hospital Anxiety and Depression Scale | C > B | |
| Line Drawing Recall Test | C > B | ||||
| Nonbinge: Nondrinkers | 7 | 6 | Paced Auditory Serial Addition Test | C > B | |
| Conditions: Some alcohol dependent subjects included, gender taken into account, alcohol intake in past week measured, only minimal smoking in subjects (<5 per day) | CANTAB–Stockings of Cambridge Test | C > B | |||
| CANTAB–IDED Test | C = B | ||||
| Bond and Lader Mood Rating Scale | C = B | ||||
| Spatial Working Memory Test | C = B | ||||
| Spatial Recognition Memory Test | C = B | ||||
| Pattern Recognition Memory Test | C = B | ||||
| Word List Recall Test | C = B | ||||
| Binge: Binge Drinking Score > cutoff | 15 | 23 | CANTAB–Matching-to-Sample Visual | C > B | |
| Nonbinge: Binge Drinking Score < cutoff | 21 | 13 | Search task: Choice Time (bingers shorter some conditions) | ||
| Conditions: No subjects with alcohol dependence, gender taken into account, subjects abstained from alcohol for >12 hr prior, smoking not controlled | CANTAB–Matching to Sample Visual | C > B | |||
| Search task: Movement Time (binger shorter) | |||||
| CANTAB-Spatial Working Memory task (F > M errors than nonbinger group) | C > B | ||||
| CANTAB-Matching to Sample Visual Search task: Errors Made | C = B | ||||
| Gordon Diagnostic System-Vigilance task | C = B | ||||
| Binge: ≥5 drinks on one occasion, ≥2 times past 30 days | Not reported | Iowa Gambling Task–Effects of Punishment Frequency | C > B (heavy bingers only) | ||
| Nonbinge: ≥5 drinks on one occasion, <2 times past 30 days | |||||
| Conditions: Groups matched on age, gender, ethnicity, smoking | |||||
Note. C = B: No significant difference between control and binge group. F = female; M = male; WMS-R = Wechsler Memory Scale-Revised; CANTAB = Cambridge Neuropsychological Test Automated Battery; IDED = Intra-Extra Dimensional Set Shift.
It is important in this context to distinguish binge drinking from alcohol dependence. For example, alcohol dependent individuals who did binge drink—that is, regularly consumed more than 10 successive drinks—were compared with an alcohol dependent group who did not binge drink. No differences in performance were found for visuo-motor speed, visuo-spatial organization/planning, learning, proactive/retroactive interference, and item retrieval efficiency ( Kokavec & Crowe, 1999 ). Comparable executive functioning results were obtained for both groups, and binge drinkers performed better than nonbinge drinkers on memory tasks. Although binge drinking was associated with impaired performance on immediate and delayed recall of verbal and visual information (Wechsler Memory Scale–Revised), retrieval ability was similar so that semantic organizational ability may be superior in binge compared with nonbinge drinkers. The pattern of binge versus nonbinge findings is likely affected by the inclusion of alcohol dependence criteria and the disproportionate number of drinks required in the binge definition.
Physiological Factors
The consensus from animal model studies is that “binge” effects require a long-term (multiple days) exposure to alcohol (e.g., Greiffenstein, Mathis, Stouwe, & Molina, 2007 ; Moore et al., 2007 ; Wezeman, Juknelis, Himes, & Callaci, 2007 )—a viewpoint similar to the clinical alcoholic binge but quite different from the most common interpretations of binge drinking discussed above. Moreover, animal studies of alcohol binge exposure have led to the conclusion that such ethanol intake can lead to neurodegeneration in corticolimbic areas linked to learning and spatial memory ( Aggleton, Hunt, & Rawlins, 1986 ; Haberly, 1998 ; Jarrard, 1993 ), such as the olfactory bulb, piriform cortex, perirhinal cortex, entorhinal cortex, and the hippocampal dentate gyrus ( Collins, Corso, & Neafsey, 1996 ; Collins, Zou, & Neafsey, 1998 ; Corso, Mostafa, Collins, & Neafsey, 1998 ; Crews, Braun, Switzer, & Knapp, 2000 ; Zou, Martinez, Neafsey, & Collins, 1996 ). Researchers have found extensive neurodegeneration of the entorhinal cortex in rats after 2 days of “binge” alcohol exposure using stomach catheters that produced learning deficits ( Obernier, White, Swartzwelder, & Crews, 2002 ). The vulnerability of this region after a single “binge” episode (i.e., 2 days of alcohol exposure) implies that long-term ethanol exposure may not produce the neurotoxicity commonly associated with heavy alcohol use. However, the duration of alcohol exposure time that leads to neurotoxicity is still unknown.
The Iowa Gambling Task (IGT) has been used to measure decision making skills in a sample of human binge (≥5 drinks on one occasion, more than one time in the past 30 days) and nonbinge alcohol drinkers. Diminished IGT performance was found in chronic high-binge drinkers (binge drinking 2 or more times a week 95% of the time) compared with low-binge drinkers (binge drinking 2 or more times a week 3% of the time). Heavy drinkers and possible alcohol dependent/abusers were included, and it was acknowledged that the findings did not permit differentiation of whether the quantity/frequency of drinking or the pattern of drinking was the cause of the diminished IGT performance ( Goudriaan, Grekin, & Sher, 2007 ).
Magnetic resonance imaging measures of regional white and gray matter regional volumes were used to quantify N-acetylaspartate (NAA) concentrations—a metabolite biomarker of neural integrity. For bingers (> 100/80 alcohol drinks/month on <21 days in the past 3 years) compared with nonbingers, decreased NAA concentrations were associated with increased metabolism and frontal white matter loss, with higher parietal gray matter NAA. Consumption amount for heavy drinkers (> 100/80 drinks per month over past 3 years, which included binge drinkers) was correlated with lower executive functioning and working memory test scores. In addition, their relative frontal NAA loss was associated with impaired executive functioning and processing speed. Taken together, the results imply that these bingers have less parietal neuron damage than continual heavy drinkers ( Meyerhoff et al., 2004 ), and that binge drinking may result in relatively specific neural deficits that differ from those associated with continual drinking levels.
Withdrawal Effects
A related issue is whether binge drinking causes permanent cognitive deficits. Previous studies of alcohol dependent adolescents suggest that frequent heavy drinking produces long-term memory deficits ( Tapert et al., 2001 ). A study of nondependent binge drinkers examined hangover effects from binge drinking (≥5 drinks on a single occasion), which were assessed with memory tasks to determine whether cognitive deficits were related to the hangover episode or long-term neural damage. Encoding and consolidation processes were impaired, but delayed recall was intact, suggesting that retrieval processes were affected only during the hangover ( Verster, van Duin, Volkerts, Schreuder, & Verbaten, 2003 ). The implications of these findings may be best described by the Federal Aviation Administration's Pilot Safety Guidelines on alcohol and flying: “eight hours from bottle to throttle” ( Salazar & Antuñano, 2008 , p. 3). Moreover, hours from last drink appear unrelated to cognitive performance ( Townshend & Duka, 2005 ), and neuropsychological impairment from heavy social drinking over 6 months has not been observed ( Alterman & Hall, 1989 ). Thus, the relationship between heavy alcohol consumption and subsequent cognitive capability is unclear.
Another interpretation suggests that increased binging causes a greater number of withdrawals, which produce the long-term deficits ( Glenn, Parsons, Sinha, & Stevens, 1988 ; Parsons & Stevens, 1986 ; Stephens et al., 2005 ). The number of alcohol withdrawals has been linked to impairments of long-term nonverbal memory in adolescents and to poor memory in adult alcoholics ( Glenn et al., 1988 ). Alcoholic patients with two or more medically supervised alcohol detoxifications demonstrated more frontal lobe cognitive dysfunction than patients with a single or no previous detoxification ( Duka, Townshend, Collier, & Stephens, 2003 ).
Neural “kindling” has been proposed as the mechanism by which alcohol ingestion and subsequent withdrawal produce cognitive damage ( Ballenger & Post, 1978 ). Repeated withdrawals are thought to generate an accumulative adaptive process that underlies the “advancing pathogenesis associated with the development of alcoholism [such that] continued alcohol abuse could be related to an avoidance of distress from worsening acute withdrawal symptoms induced by a kindling process that advances the course of alcoholism” ( Breese, Overstreet, & Knapp, 2005 , pp. 371–372). This view is consistent with an increased risk for brain damage from binge drinking and subsequent withdrawal ( Hunt, 1993 ; Wechsler et al., 1994 ).
The occurrence of “blackouts” in which complex activities are performed with no recollection of the behavior available may be a related phenomenon and perhaps a biomarker for the mechanism of neurotoxicity observed in binge drinkers. Blackouts occur often in binge drinkers and could originate from reduced activity of N-methyl-D-aspartate (NMDA) receptors in the hippocampus, which would impair long-term potentiation ( Izumi, Nagashima, Murayama, & Zorumski, 2005 ; for a review, see Allgaier, 2002 ). Excessive glucocorticoid release induced by the withdrawal stress could intensify the responses of already overactive NMDA receptors, thereby initiating blackouts ( Hunt, 1993 ). Periods of binging followed by abstinence then trigger a neural cycle that leads to increased neurotoxicity of structures involved in learning and memory.
Alcoholism, Alcohol Dependence, and Other Determinants
Table 3 summarizes the definitions of alcohol abuse and dependence from the Diagnostic and Statistical Manual of Mental Disorders (4th ed.; American Psychiatric Association, 1994 ). Inclusion of frank alcoholism in binge samples may result in biased drinking correlates stemming from the negative consequences of alcoholism as well as binging. Alcohol dependence also can alter binge-drinking outcomes. College students who are frequent heavy episodic drinkers (5/4 or more drinks on three or more occasions in the past 2 weeks) had 19 times greater odds of being classified with alcohol dependence and 13 times greater odds of being classified with alcohol abuse compared with nonheavy episodic drinkers. The occasional episodic drinkers (heavy drinking on one or two occasions during the past 2 weeks) were found to have 4 times greater odds of dependence or abuse compared with the nonheavy episodic drinkers ( Knight et al., 2002 ). However, earlier reports suggest that the comorbidity of binge drinking (periodic heavy drinking followed by a period of abstinence), with alcohol addiction or dependence, is not clinically supported ( Levy, 1988 ; Levy & Kunitz, 1974 ).
| Binge drinking | Alcohol abuse | Dependence |
|---|---|---|
| : Pattern of drinking alcohol that brings BAC to 0.08 gram % and above (approximately equivalent to the consumption of 5 drinks for men, 4 for women, in 2 hr) | A maladaptive pattern of substance use leading to clinically significant impairment or distress, as manifested by one (or more) of the following, occurring within a 12-month period: | A maladaptive pattern of substance use, leading to clinically significant impairment or distress, as manifested by three (or more) of the following, occurring at any time in the same 12-month period: |
| Proposed: Pattern of drinking alcohol that brings BAC to 0.08 gram % and above (approximately equivalent to the consumption of 5 drinks for males, 4 for females, in 2 hours), occurring more than once within a 6-month period. |
Note. NIAA = National Institute on Alcohol Abuse and Alcoholism; BAC = blood alcohol concentration.
Parental history for alcoholism and binge drinking (≥5 drinks per occasion) in a sample of alcohol dependent individuals both have been found to influence short-term outcome of alcohol dependence ( Hasin, Paykin, & Endicott, 2001 ). An additional factor is gender, because as many as 81% of all binge-drinking episodes are attributed to men ( Naimi et al., 2003 ), but men also demonstrate increased frequency of alcohol dependence ( Robin, Long, Rasmussen, Albaugh, & Goldman, 1998 ). These data suggest that the relationships among binge-drinking definitions, epidemiological findings, and alcohol-related diagnostic categories need additional refinement.
Family History
Presence of alcoholism in the family covaries with behavioral and neuroimaging measures of binge drinking ( Ehlers et al., 2007 ; Kokavec & Crowe, 1999 ). Alcohol expectancies have been shown to be a genetically influenced characteristic having a heritability between 0.4 and 0.6 ( Heath et al., 1999 ; Schuckit et al., 2001 ), with greater alcohol consumption in high-risk than in low-risk control families ( Newlin & Thomson, 1990 ). After the consumption of the lower or higher ethanol dose (approximately three or five drinks, respectively), men with high risk for alcoholism reported significantly less intense feelings of intoxication compared with low-risk men ( Ehlers & Schuckit, 1988 ; O'Malley & Maisto, 1988 ; Schuckit, 1980 , 1984 , 1988 ). As outlined above, individuals who are homozygous for the ALDH2 gene are less likely to binge drink ( Luczak, Wall, Shea, Byun, & Carr, 2001 ), which needs to be considered in such studies.
These associations have spurred the search for a binge-drinking gene. College students with the short version of the serotonin transporter gene (5-HTT) consumed more alcohol per occasion, more often drank expressly to become inebriated, and were more likely to engage in binge drinking than college students without the 5-HTT variant ( Herman, Philbeck, Vasilopoulos, & Depetrillo, 2003 ). The 5-HTT gene is thought to be involved in serotonin reuptake, and the students who were homozygotic for the short version of 5-HTT were more likely to report troublesome drinking patterns. Students with at least one copy of the 5-HTT long variant gene consume fewer alcoholic drinks per episode but are equal in the number of episodes. Individuals who are homozygous for the short version are also at risk for higher levels of anxiety and depression and may use alcohol to reduce tension ( Mazzanti et al., 1998 ).
Event-Related Potentials (ERPs)
ERPs are sensitive to the neural effects of alcohol intake ( Porjesz & Begleiter, 1996 ). Several studies have reported decreases in ERP component (N1, MMN, P300) amplitudes with ethanol doses ranging from 0.50 g/kg to 0.85 g/kg ( Campbell & Lowick, 1987 ; Grillon, Sinha, & O'Malley, 1995 ; Jääskeläinen et al., 1995 , 1998 ; Rohrbaugh et al., 1987 ; Sommer, Leuthold, & Hermanutz, 1993 ). The P300 component reflects attention and memory operations engaged when stimulus change occurs ( Polich, 2007 ). P300 variation with ethanol ingestion has been interpreted as demonstrating adverse effects on perceptual processing resources, a measure of central nervous system disinhibition, or frontal executive dysfunction ( Begleiter & Porjesz, 1999 ; George, Potts, Kothman, Martin, & Mukundan, 2004 ; Kim, Kim, & Kwon, 2001 ).
ERPs also have been used to assess familial history as a neural signature or “marker” of alcoholism ( Begleiter, Porjesz, Bihari, & Kissin, 1984 ; Hill et al., 1998 ; Hill & Steinhauer, 1993 ; O'Connor, Hesslebrock, Tasman, & DePalma, 1987 ; Porjesz & Begleiter, 1990 ). A meta-analysis of the early studies found that these effects were variable ( Polich, Pollock, & Bloom, 1994 ), and that difficult visual discrimination tasks produced the strongest family history effects (e.g., Carlson, Iacono, & McGue, 2002 ; Iacono, Carlson, Malone, & McGue, 2002 ; Reese & Polich, 2003 ). These findings suggest that the P300 component in particular can index the effects of alcohol intake and may reflect the genetic background of alcoholism.
ERPs are just beginning to be used to assay binge drinking. A facial discrimination task yielded P300 amplitudes that were smaller for adolescents exposed to alcohol (i.e., ≥5 drinks per occasion), with a positive family history for alcohol dependence acting as a significant covariate. Further, P300 latency was decreased for alcohol and drug-exposed young adults in the absence of an alcohol challenge relative to control participants ( Ehlers et al., 2007 ). Recent ERP studies suggest that high-binge compared with low-binge college student groups can be differentiated with tasks requiring strong visual stimulus processing: P300 amplitude tends to be smaller for the high- compared with the low-binge groups, although the quantity and frequency of alcohol intake that produces these effects are still unclear ( Courtney & Polich, 2008 ).
The present review highlights issues that contribute to the definition of binge drinking, with the main variables centering on the quantity consumed and the time-frame of consumption. However, alcohol consumption effects are modulated by individual variation with respect to expectancy, how expectations influence the perception of inebriation, tolerance to alcohol ingestion, and the social environment. These factors contribute to the characterization of binge drinking in relation to its cognitive, physiological, and withdrawal effects. Moreover, the relevant findings empirically differentiate binge drinking from clinical alcoholism by defining how these variables influence alcohol effects. Thus, the interactive milieu of alcohol's internal determinants is complex and surprisingly subtle, so that binging to some is not necessarily binging to others.
An Operational Definition
Epidemiological reports of binge drinking vary in definitional consistency, but for young adults they indicate a large prevalence and imply a clear burden of suffering. The individual and social costs associated with binge drinking—such as drunken driving, induced violence, and personal injury—are profound. The cognitive damage that may be inflicted by binge drinking appears to involve alteration in critical neural mechanisms. However, experimental binge-drinking studies vary in their definitional approaches so that the what, where, and when of the neurocognitive insult is uncertain. Functional magnetic resonance imaging and ERP methods are beginning to assay such outcomes, but these approaches require sustained definitional rigor to inform public health policies.
The current NIAAA (2004) definition has provided a structure for binge drinking, but scientific and clinical assessments would benefit from the formation of a definition that facilitates comparison among studies. Given the findings outlined above, this definition should encompass three factors: alcohol quantity consumed, time-frame of consumption, and time period of past binging episodes. A definition of binge drinking that integrates these issues is as follows: A pattern of drinking alcohol that brings BAC to 0.08 gram percent or above (≥5/4 for men/women in 2 hr) on more than one occasion within the past 6 months . This definition (1) is operational in structure, (2) delimits consumption amount and time-frame (taking into account gender), and (3) specifies a time period that encompasses individual variation.
Future Directions
The intriguing hints provided by initial genetic studies may ultimately identify the neural origins of propensity to binge drink, which likely reflect fundamental individual differences to alcohol intake and interact with the wider context of personality or psychiatric variables. Searching for the primary reasons why some young adults binge would foster genetic links between binge drinking and subsequent alcohol dependence. Characterizing the association between binge-drinking mechanisms and the development of alcoholism could reveal a means to pursue and evaluate treatment interventions before the addictive disease is fully developed.
Neurophysiological and neurocognitive assessments of binge drinking are demonstrating promise in specifying biological differences between bingers and controls. The biphasic alcohol response exhibited by young binge drinkers and the associated neuropsychological impairments found for frontal lobe processing provide clues to the origins of binge drinking. Preliminary findings suggest working memory deficits in binge drinkers, but whether these are long-term or abate after withdrawal is unknown. Although difficult to execute, longitudinal studies of adolescent binge drinking could establish whether and how future alcohol dependence and abuse originates from this pattern of alcohol consumption while controlling for family history. Addressing these issues with a quantifiable and consistent binge-drinking definition would encourage comparisons among studies and increase their societal impact.
Scientific understanding of how alcohol produces reactions that vary across individuals from pleasurable to deadly requires clear observation of the phenomena and definitional agreement about what is observed. The public health concerns about young adult binge drinking have helped to motivate refinement of its definition. The implications of the empirical framework outlined here can be used to evaluate the proposed quantities, time-frame, and consumption frequencies as factors that may contribute to subsequent alcohol-related problems. The proposed binge-drinking definition should therefore help provide the operational utility that will facilitate inferences across studies.
Acknowledgments
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grant AG10604. This article is 19458-MIND (Molecular and Integrative Neuroscience Department) from The Scripps Research Institute. We thank Shirley Y. Hill and Brian Lopez for very helpful comments on earlier versions of this article.
Contributor Information
Kelly E. Courtney, Department of Psychology, San Diego State University, La Jolla, California.
John Polich, Molecular and Integrative Neurosciences Department, The Scripps Research Institute, La Jolla, California.
- Aggleton JP, Hunt PR, Rawlins JN. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behavioural Brain Research. 1986; 19 :133–146. [ PubMed ] [ Google Scholar ]
- Alcohol Policy Information System. Adult operator blood alcohol concentration limit laws as of January 1, 2007. 2007. Retrieved July 07, 2008, from http://www.alcoholpolicy.niaaa.nih.gov/index.asp?Type=BAS_APIS&SEC={85AC5A78–16B8–444A–95BE-BA2A10EEE53A}&DE={9F60E063–02C1–46B2–8AF0–8F" .
- Allgaier C. Ethanol sensitivity of NMDA receptors. Neurochemistry International. 2002; 41 :377–382. [ PubMed ] [ Google Scholar ]
- Alterman AI, Hall JG. Effects of social drinking and familial alcoholism risk on cognitive functioning: Null findings. Alcoholism: Clinical and Experimental Research. 1989; 13 :799–803. [ PubMed ] [ Google Scholar ]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: Author; 1994. [ Google Scholar ]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. British Journal of Psychiatry. 1978; 133 :1–14. [ PubMed ] [ Google Scholar ]
- Baum-Baicker C. The psychological benefits of moderate alcohol consumption: A review of the literature. Drug and Alcohol Dependence. 1985; 15 :305–322. [ PubMed ] [ Google Scholar ]
- Begleiter HH, Porjesz BB. What is inherited in the predisposition toward alcoholism? A proposed model. Alcoholism: Clinical and Experimental Research. 1999; 23 :1125–1135. [ PubMed ] [ Google Scholar ]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984 September 28; 225 :1492–1496. [ PubMed ] [ Google Scholar ]
- Blekher T, Beard JD, O'Connor S, Orr WE, Ramchandani VA, Miller K, et al. Response of saccadic eye movements to alcohol in African American and non-Hispanic White college students. Alcoholism: Clinical and Experimental Research. 2002; 26 :232–238. [ PubMed ] [ Google Scholar ]
- Blume AW, Schmaling KB, Marlatt AG. Predictors of change in binge drinking over a 3-month period. Addictive Behaviors. 2003; 28 :1007–1012. [ PubMed ] [ Google Scholar ]
- Boyd CJ, McCabe SE, Morales M. College students' alcohol use: A critical review. Annual Review of Nursing Research. 2005; 23 :179–211. [ PubMed ] [ Google Scholar ]
- Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiology of alcoholism: A “kindling”/stress hypothesis. Psychopharmacology. 2005; 178 :367–380. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cahalan D, Cisin IH, Crossley HM. American drinking practices: A national study of drinking behavior and attitudes. New Brunswick, NJ: Rutgers Center of Alcohol Studies; 1969. [ Google Scholar ]
- Campbell KB, Lowick BM. Ethanol and event-related potentials: The influence of distractor stimuli. Alcohol. 1987; 4 :257–263. [ PubMed ] [ Google Scholar ]
- Carlson SR, Iacono WG, McGue M. P300 amplitude in adolescent twins discordant and concordant for alcohol use disorders. Biological Psychology. 2002; 61 :203–227. [ PubMed ] [ Google Scholar ]
- Chait LD, Perry JL. Acute and residual effects of alcohol and marijuana, alone and in combination, on mood and performance. Psychopharmacology. 1994; 115 :340–349. [ PubMed ] [ Google Scholar ]
- Chikritzhs TN, Jonas HA, Stockwell TR, Heale PF, Dietze PM. Mortality and life-years lost due to alcohol: A comparison of acute and chronic causes. Medical Journal of Australia. 2001; 174 :281–284. [ PubMed ] [ Google Scholar ]
- Christiansen BA, Goldman MS. Alcohol-related expectancies versus demographic/background variables in the prediction of adolescent drinking. Journal of Consulting and Clinical Psychology. 1983; 51 :249–257. [ PubMed ] [ Google Scholar ]
- Clapp JD, Lange J, Min JW, Shillington A, Johnson M, Voas R. Two studies examining environmental predictors of heavy drinking by college students. Prevention Science. 2003; 4 :99–108. [ PubMed ] [ Google Scholar ]
- Clapp JD, Shillington AM. Environmental predictors of heavy episodic drinking events. American Journal of Drug and Alcohol Abuse. 2001; 27 :310–313. [ PubMed ] [ Google Scholar ]
- Collins MA, Corso TD, Neafsey EJ. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic “binge” intoxication with ethanol: Possible explanation for olfactory deficits in alcoholics. Alcoholism: Clinical and Experimental Research. 1996; 20 :284–292. [ PubMed ] [ Google Scholar ]
- Collins MA, Zou JY, Neafsey EJ. Brain damage due to episodic alcohol exposure in vivo and in vitro: Furosemide neuroprotection implicates edema-based mechanism. The FASEB Journal. 1998; 12 :221–230. [ PubMed ] [ Google Scholar ]
- Cook TA, Luczak SE, Shea SH, Ehlers CL, Carr LG, Wall TL. Associations of ALDH2 and ADH1B genotypes with response to alcohol in Asian Americans. Journal of Studies on Alcohol. 2005; 66 :196–204. [ PubMed ] [ Google Scholar ]
- Corso TD, Mostafa HM, Collins MA, Neafsey EJ. Brain neuronal degeneration caused by episodic alcohol intoxication in rats: Effects of nimodipine, 6,7-dinitro-quinoxaline-2,3-dione, and MK-801. Alcoholism: Clinical and Experimental Research. 1998; 22 :217–224. [ PubMed ] [ Google Scholar ]
- Courtney KE, Polich J. Neuroelectric assessment of binge drinking [Abstract] Psychophysiology. 2008; 45 :S50. [ Google Scholar ]
- Cranford JA, McCabe SE, Boyd CJ. A new measure of binge drinking: Prevalence and correlates in a probability sample of undergraduates. Alcoholism: Clinical and Experimental Research. 2006; 30 :1896–1905. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Crews FT, Braun CJ, Switzer RC, Knapp DJ. Binge ethanol causes differential brain damage in young adolescent rats compared to adult rats. Alcoholism: Clinical and Experimental Research. 2000; 24 :1712–1723. [ PubMed ] [ Google Scholar ]
- Dawson DA, Grant BF, Stinson FS, Chou PS. Another look at heavy episodic drinking and alcohol use disorders among college and noncollege youth. Journal of Studies on Alcohol. 2004; 65 :477–488. [ PubMed ] [ Google Scholar ]
- Duka T, Townshend JM, Collier K, Stephens DN. Impairment in cognitive functions after multiple detoxifications in alcoholic inpatients. Alcoholism: Clinical and Experimental Research. 2003; 27 :1563–1572. [ PubMed ] [ Google Scholar ]
- Ehlers CL, Phillips E, Finnerman G, Gilder D, Lau P, Criado J. P3 components and adolescent binge drinking in Southwest California Indians. Neurotoxicology and Teratology. 2007; 29 :153–163. [ PubMed ] [ Google Scholar ]
- Ehlers CL, Schuckit MA. EEG response to ethanol in sons of alcoholics. Psychopharmacology Bulletin. 1988; 24 :434–437. [ PubMed ] [ Google Scholar ]
- Fillmore MT, Vogel-Sprott M. Behavioral impairment under alcohol: Cognitive and pharmacokinetic factors. Alcoholism: Clinical and Experimental Research. 1998; 22 :1476–1482. [ PubMed ] [ Google Scholar ]
- Fogarty JN, Vogel-Sprott M. Cognitive processes and motor skills differ in sensitivity to alcohol reinforcement. Journal of Studies on Alcohol. 2002; 63 :404–411. [ PubMed ] [ Google Scholar ]
- Fromme K, Marlatt GA, Baer JS, Kivlahan DR. The Alcohol Skills Training Program: A group intervention for young adult drinkers. Journal of Substance Abuse Treatment. 1994; 11 :143–154. [ PubMed ] [ Google Scholar ]
- George MR, Potts G, Kothman D, Martin L, Mukundan CR. Frontal deficits in alcoholism: An ERP study. Brain and Cognition. 2004; 54 :245–247. [ PubMed ] [ Google Scholar ]
- Glenn SW, Parsons OA, Sinha R, Stevens L. The effects of repeated withdrawals from alcohol on the memory of male and female alcoholics. Alcohol and Alcoholism. 1988; 23 :337–342. [ PubMed ] [ Google Scholar ]
- Goldstein AL, Wall A, McKee SA, Hinson RE. Accessibility of alcohol expectancies from memory: Impact of mood and motives in college drinkers. Journal of Studies on Alcohol. 2004; 65 :95–104. [ PubMed ] [ Google Scholar ]
- Goudriaan AE, Grekin ER, Sher KJ. Decision making and binge drinking: A longitudinal study. Alcoholism: Clinical and Experimental Research. 2007; 31 :928–938. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Greiffenstein P, Mathis KW, Stouwe CV, Molina PE. Alcohol binge before trauma/hemorrhage impairs integrity of host defense mechanisms during recovery. Alcoholism: Clinical and Experimental Research. 2007; 31 :704–715. [ PubMed ] [ Google Scholar ]
- Grillon C, Sinha R, O'Malley SS. Effects of ethanol on the processing of low probability stimuli: An ERP study. Psychopharmacology. 1995; 119 :455–465. [ PubMed ] [ Google Scholar ]
- Haberly L. The synaptic organization of the brain. In: Shepherd GM, editor. Olfactory cortex. New York: Oxford University Press; 1998. pp. 377–416. [ Google Scholar ]
- Hartley DE, Elsabagh S, File SE. Binge drinking and sex: Effects on mood and cognitive function in healthy young volunteers. Pharmacology, Biochemistry and Behavior. 2004; 78 :611–619. [ PubMed ] [ Google Scholar ]
- Hasin SS, Paykin A, Endicott J. Course of DSM–IV alcohol dependence in a community sample: Effects of parental history and binge drinking. Alcoholism: Clinical and Experimental Research. 2001; 25 :411–414. [ PubMed ] [ Google Scholar ]
- Heath AC, Madden PA, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, et al. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychological Medicine. 1999; 29 :1069–1081. [ PubMed ] [ Google Scholar ]
- Heishman SJ, Arasteh K, Stitzer ML. Comparative effects of alcohol and marijuana on mood, memory, and performance. Pharmacology, Biochemistry and Behavior. 1997; 58 :93–101. [ PubMed ] [ Google Scholar ]
- Herman AI, Philbeck JW, Vasilopoulos NL, Depetrillo PB. Serotonin transporter promoter polymorphism and differences in alcohol consumption behavior in a college student population. Alcohol and Alcoholism. 2003; 38 :446–449. [ PubMed ] [ Google Scholar ]
- Hill SY, Locke J, Zezza N, Kaplan B, Neiswanger K, Steinhauer SR, et al. Genetic association between reduced P300 amplitude and the DRD2 dopamine receptor A1 allele in children at high risk for alcoholism. Biological Psychiatry. 1998; 43 :40–51. [ PubMed ] [ Google Scholar ]
- Hill SY, Shen S, Lowers L, Locke J. Factors predicting the onset of adolescent drinking in families at high risk for developing alcoholism. Biological Psychiatry. 2000; 48 :265–275. [ PubMed ] [ Google Scholar ]
- Hill SY, Steinhauer S. Assessment of prepubertal and postpubertal boys and girls at risk for developing alcoholism with P300 from a visual discrimination task. Journal of Studies on Alcohol. 1993; 54 :350–358. [ PubMed ] [ Google Scholar ]
- Holdstock L, de Wit H. Ethanol impairs saccadic and smooth pursuit eye movements without producing self-reports of sedation. Alcoholism, Clinical and Experimental Research. 1999; 23 :664–672. [ PubMed ] [ Google Scholar ]
- Holdstock L, King AC, de Wit H. Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcoholism: Clinical and Experimental Research. 2000; 24 :789–794. [ PubMed ] [ Google Scholar ]
- Hunt WA. Are binge drinkers more at risk of developing brain damage? Alcohol. 1993; 10 :559–561. [ PubMed ] [ Google Scholar ]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Archives of General Psychiatry. 2002; 59 :750–751. [ PubMed ] [ Google Scholar ]
- Izumi Y, Nagashima K, Murayama K, Zorumski CF. Acute effects of ethanol on hippocampal long-term potentiation and long-term depression are mediated by different mechanisms. Neuroscience. 2005; 136 :509–517. [ PubMed ] [ Google Scholar ]
- Jääskeläinen IP, Hirvonen J, Kujala T, Alho K, Eriksson CJ, Lehtokoski A, et al. Effects of naltrexone and ethanol on auditory event-related brain potentials. Alcohol. 1998; 15 :105–111. [ PubMed ] [ Google Scholar ]
- Jääskeläinen IP, Lehtokoski A, Alho K, Kujala T, Pekkonen E, Sinclair JD, et al. Low dose of ethanol suppresses mismatch negativity of auditory event-related potentials. Alcoholism: Clinical and Experimental Research. 1995; 19 :607–610. [ PubMed ] [ Google Scholar ]
- Jaccard J, Turrisi R. Cognitive processes and individual differences in judgments relevant to drunk driving. Journal of Personality and Social Psychology. 1987; 53 :135–145. [ PubMed ] [ Google Scholar ]
- Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behavioral and Neural Biology. 1993; 60 :9–26. [ PubMed ] [ Google Scholar ]
- Kim MS, Kim JJ, Kwon JS. Frontal P300 decrement and executive dysfunction in adolescents with conduct problems. Child Psychiatry and Human Development. 2001; 32 :93–106. [ PubMed ] [ Google Scholar ]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcoholism: Clinical and Experimental Research. 2002; 26 :827–835. [ PubMed ] [ Google Scholar ]
- Knight JR, Wechsler H, Kuo M, Seibring M, Weitzman ER, Schuckit MA. Alcohol abuse and dependence among U.S. college students. Journal of Studies on Alcohol. 2002; 63 :263–270. [ PubMed ] [ Google Scholar ]
- Kokavec A, Crowe SF. A comparison of cognitive performance in binge versus regular chronic alcohol misusers. Alcohol and Alcoholism. 1999; 34 :601–608. [ PubMed ] [ Google Scholar ]
- LaBrie J, Pedersen ER, Tawalbeh S. Classifying risky-drinking college students: Another look at the two-week drinker-type categorization. Journal of Studies on Alcohol and Drugs. 2007; 68 :1–5. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lange JE, Voas RB. Defining binge drinking quantities through resulting BACs. Annual Proceedings/Association for the Advancement of Automotive Medicine. 2000a; 44 :389–404. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lange JE, Voas RB. Youth escaping limits on drinking: Binging in Mexico. Addiction. 2000b; 95 :521–528. [ PubMed ] [ Google Scholar ]
- Levy JE. The effects of labeling on health behavior and treatment programs among North American Indians [Monograph] American Indian and Alaska Native Mental Health Research. 1988; 1 :211–231. [ PubMed ] [ Google Scholar ]
- Levy JE, Kunitz SJ. Indian drinking: Navajo practices and Anglo-American theories. New York: Wiley; 1974. [ Google Scholar ]
- Luczak SE, Wall TL, Shea SH, Byun SM, Carr LG. Binge drinking in Chinese, Korean, and White college students: Genetic and ethnic group differences. Psychology of Addictive Behaviors. 2001; 15 :306–309. [ PubMed ] [ Google Scholar ]
- Mattila MJ, Patat A, Seppälä T, Kalska H, Jalava ML, Vanakoski J, et al. Single oral doses of amisulpride do not enhance the effects of alcohol on the performance and memory of healthy subjects. European Journal of Clinical Pharmacology. 1996; 51 :161–166. [ PubMed ] [ Google Scholar ]
- Mazzanti CM, Lappalainen J, Long JC, Bengel D, Naukkarinen H, Eggert M, et al. Role of the serotonin transporter promoter polymorphism in anxiety-related traits. Archives of General Psychiatry. 1998; 55 :936–940. [ PubMed ] [ Google Scholar ]
- McGinnis JM, Foege WH. Actual causes of death in the United States. Journal of the American Medical Association. 1993; 270 :2207–2212. [ PubMed ] [ Google Scholar ]
- Mehrabian A, Russell JA. A questionnaire measure of habitual alcohol use. Psychological Reports. 1978; 43 :803–806. [ PubMed ] [ Google Scholar ]
- Meyerhoff DJ, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, et al. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcoholism: Clinical and Experimental Research. 2004; 28 :650–661. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Miller JW, Naimi TS, Brewer RD, Jones SE. Binge drinking and associated health risk behaviors among high school students. Pediatrics. 2007; 119 (1):76–85. [ PubMed ] [ Google Scholar ]
- Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL., II GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacology, Biochemistry and Behavior. 2007; 88 :105–113. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Moser A, Heide W, Kömpf D. The effects of oral ethanol consumption on eye movements in healthy volunteers. Journal of Neurology. 1998; 245 :542–559. [ PubMed ] [ Google Scholar ]
- Naimi TS, Brewer RD, Mokdad A, Denny C, Serdula MK, Marks JS. Binge drinking among U.S. adults. Journal of the American Medical Association. 2003; 289 :70–75. [ PubMed ] [ Google Scholar ]
- National Institute on Alcohol Abuse and Alcoholism. Tenth special report to the US Congress on alcohol and health. Bethesda MD: National Institutes of Health; 2000. [ Google Scholar ]
- National Institute on Alcohol Abuse and Alcoholism. National Institute of Alcohol Abuse and Alcoholism Council approves definition of binge drinking. NIAAA Newsletter. 2004. Winter. Retrieved October 25, 2007, from http://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.htm .
- Nelson TO, Graf A, Dunlosky J, Marlatt A, Walker D, Luce K. Effect of acute alcohol intoxication on recall and on judgments of learning during the acquisition of new information. In: Mazzoni G, Nelson TO, editors. Metacognition and cognitive neuropsychology: Monitoring and control processes. Mahwah, NJ: Erlbaum; 1998. pp. 161–180. [ Google Scholar ]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: A critical review and analysis. Psychological Bulletin. 1990; 108 :383–402. [ PubMed ] [ Google Scholar ]
- Nichols JM, Martin F. The effect of lorazepam on long-term verbal recall in heavy and light social drinkers. Alcohol. 1997; 14 :455–461. [ PubMed ] [ Google Scholar ]
- Obernier JA, White AM, Swartzwelder HS, Crews FT. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacology, Biochemistry and Behavior. 2002; 72 :521–532. [ PubMed ] [ Google Scholar ]
- O'Connor S, Hesslebrock V, Tasman A, DePalma N. P3 amplitude in two distinct tasks is decreased in young men with a history of paternal alcoholism. Alcohol. 1987; 4 :323–330. [ PubMed ] [ Google Scholar ]
- Oei TPS, Morawska A. A cognitive model of binge drinking: The influence of alcohol expectancies and drinking refusal self-efficacy. Addictive Behaviors. 2004; 29 :159–179. [ PubMed ] [ Google Scholar ]
- Okoro CA, Brewer RD, Naimi TS, Moriarty DG, Giles WH, Mokdad AH. Binge drinking and health-related quality of life: Do popular perceptions match reality? American Journal of Preventive Medicine. 2004; 26 :230–233. [ PubMed ] [ Google Scholar ]
- O'Malley SS, Maisto SA. Effects of family drinking history and expectancies on responses to alcohol in men. Journal of Studies on Alcohol. 1988; 46 :289–297. [ PubMed ] [ Google Scholar ]
- Parsons OA, Stevens L. Previous alcohol intake and residual cognitive deficits in detoxified alcoholics and animals. Alcohol and Alcoholism. 1986; 21 :137–157. [ PubMed ] [ Google Scholar ]
- Peele S, Brodsky A. Exploring psychological benefits associated with moderate alcohol use: A necessary corrective to assessments of drinking outcomes? Drug and Alcohol Dependence. 2000; 60 :221–247. [ PubMed ] [ Google Scholar ]
- Pernanen K. Alcohol in human violence. New York: Guilford Press; 1991. [ Google Scholar ]
- Pliner P, Cappell H. Modification of affective consequences of alcohol: A comparison of social and solitary drinking. Journal of Abnormal Psychology. 1974; 83 :418–425. [ PubMed ] [ Google Scholar ]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology. 2007; 118 :2128–2148. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Polich J, Pollock V, Bloom FE. Meta-analysis of P300 amplitude from males at-risk for alcoholism. Psychological Bulletin. 1994; 115 :55–73. [ PubMed ] [ Google Scholar ]
- Porjesz B, Begleiter H. Event-related potentials in individuals at risk for alcoholism. Alcohol. 1990; 7 :465–469. [ PubMed ] [ Google Scholar ]
- Porjesz B, Begleiter H. Effects of alcohol on electrophysiological activity of the brain. In: Begleiter H, Kissin B, editors. The pharmacology of alcohol and alcohol dependence. 2nd. New York: Oxford University Press; 1996. pp. 207–247. [ Google Scholar ]
- Presley CA, Pimentel ER. The introduction of heavy and frequent drinker: A proposed classification to increase accuracy of alcohol assessments in postsecondary educational settings. Journal of Studies on Alcohol. 2006; 67 :324–332. [ PubMed ] [ Google Scholar ]
- Read JP, Beattie M, Chamberlain R, Merrill JE. Beyond the “binge” threshold: Heavy drinking patterns and their association with alcohol involvement indices in college students. Addictive Behaviors. 2008; 33 :225–234. [ PubMed ] [ Google Scholar ]
- Reese C, Polich J. Alcoholism risk and the P300 event-related brain potential: Modality, task, and gender effects. Brain and Cognition. 2003; 53 :46–57. [ PubMed ] [ Google Scholar ]
- Robin RW, Long JC, Rasmussen JK, Albaugh B, Goldman D. Relationship of binge drinking to alcohol dependence, other psychiatric disorders, and behavioral problems in an American Indian tribe. Alcoholism: Clinical and Experimental Research. 1998; 22 :518–523. [ PubMed ] [ Google Scholar ]
- Rohrbaugh JW, Stapleton JM, Parasuraman R, Zubovic EA, Frowein HW, Varner JL, et al. Dose-related effects of ethanol on visual sustained attention and event-related potentials. Alcohol. 1987; 4 :293–300. [ PubMed ] [ Google Scholar ]
- Ross DF, Pihl RO. Alcohol, self-focus and complex reaction-time performance. Journal of Studies on Alcohol. 1988; 49 :115–125. [ PubMed ] [ Google Scholar ]
- Sacco RL, Elkind M, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, et al. The protective effect of moderate alcohol consumption on ischemic stroke. Journal of the American Medical Association. 1999; 281 :53–60. [ PubMed ] [ Google Scholar ]
- Salazar GJ, Antunano MJ. Alcohol and flying: A deadly combination. 2008. Retrieved March 17, 2008, http://www.faa.gov/pilots/safety/pilotsafetybrochures/media/alcohol.pdf .
- Sayette MA. Psychological theories of drinking and alcoholism. In: Leonard KE, Blane HT, editors. Cognitive theory and research. 2nd. New York: Guilford Press; 1999. pp. 247–291. [ Google Scholar ]
- Schuckit MA. Self-rating of alcohol intoxication by young men with and without family histories of alcoholism. Journal of Studies on Alcohol. 1980; 41 :242–249. [ PubMed ] [ Google Scholar ]
- Schuckit MA. Subjective response to alcohol in sons of alcoholics and controls. Archives of General Psychiatry. 1984; 41 :879–884. [ PubMed ] [ Google Scholar ]
- Schuckit MA. Reactions to alcohol in sons of alcoholics and controls. Alcoholism: Clinical and Experimental Research. 1988; 12 :465–470. [ PubMed ] [ Google Scholar ]
- Schuckit MA, Edenberg HJ, Kalmijn J, Flury L, Smith TL, Reich T, et al. A genome-wide search for genes that relate to a low level of response to alcohol. Alcoholism: Clinical and Experimental Research. 2001; 25 :323–329. [ PubMed ] [ Google Scholar ]
- Schulenberg J, O'Malley PM, Bachman JG, Wadsworth KN, Johnston LD. Getting drunk and growing up: Trajectories of frequent binge drinking during the transition to young adulthood. Journal of Studies on Alcohol. 1996; 57 :289–304. [ PubMed ] [ Google Scholar ]
- Slutske WS. Alcohol use disorders among U.S. college students and their non-college-attending peers. Archives of General Psychiatry. 2005; 62 :321–327. [ PubMed ] [ Google Scholar ]
- Sommer W, Leuthold H, Hermanutz M. Covert effects of alcohol revealed by event-related potentials. Perception & Psychophysics. 1993; 54 :127–135. [ PubMed ] [ Google Scholar ]
- Stephens DN, Ripley TL, Borlikova G, Schubert M, Albrecht D, Hogarth L, et al. Repeated ethanol exposure and withdrawal impairs human fear conditioning and depresses long-term potentiation in rat amygdala and hippocampus. Biological Psychiatry. 2005; 58 :392–400. [ PubMed ] [ Google Scholar ]
- Storm T, Cutler RE. Observations of drinking in natural settings: Vancouver beer parlors and cocktail lounges. Journal of Studies on Alcohol. 1981; 42 :972–997. [ PubMed ] [ Google Scholar ]
- Substance Abuse and Mental Health Services Administration. Results from the 2006 National Survey on Drug Use and Health: National findings (Office of Applied Studies, NSDUH Series H-32, DHHS Publication No SMA 07–4293) Rockville, MD: Author; 2007. [ Google Scholar ]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcoholism: Clinical and Experimental Research. 2001; 25 :236–245. [ PubMed ] [ Google Scholar ]
- Tomsovic M. “Binge” and continuous drinkers: Characteristics and treatment follow-up. Quarterly Journal of Studies on Alcohol. 1974; 35 :558–564. [ PubMed ] [ Google Scholar ]
- Townshend JM, Duka T. Patterns of alcohol drinking in a population of young social drinkers: A comparison of questionnaire and diary measures. Alcohol and Alcoholism. 2002; 37 :187–192. [ PubMed ] [ Google Scholar ]
- Townshend JM, Duka T. Binge drinking, cognitive performance and mood in a population of young social drinkers. Alcoholism: Clinical and Experimental Research. 2005; 29 :317–325. [ PubMed ] [ Google Scholar ]
- Turrisi R, Wiersma K. Examination of judgments of drunkenness, binge drinking, and drunk-driving tendencies in teens with and without a family history of alcohol abuse. Alcoholism: Clinical and Experimental Research. 1999; 23 :1191–1198. [ PubMed ] [ Google Scholar ]
- Verster JC, van Duin D, Volkerts ER, Schreuder AH, Verbaten MN. Alcohol hangover effects on memory functioning and vigilance performance after an evening of binge drinking. Neuropsychopharmacology. 2003; 28 :740–746. [ PubMed ] [ Google Scholar ]
- Vik PW, Tate SR, Carrello P. Detecting college binge drinkers using an extended time frame. Addictive Behaviors. 2000; 25 :607–612. [ PubMed ] [ Google Scholar ]
- Wechsler H. Binge drinking: Should we attack the name or the problem? [Electronic version] The Chronicle of Higher Education, B12. 2000. Oct 20, Retrieved October 15, 2007, from http://chronicle.com/weekly/v47/i08/08b01201.htm .
- Wechsler H, Davenport A, Dowdall G, Moeykens B, Castillo S. Health and behavioral consequences of binge drinking in college: A national survey of students at 140 campuses. Journal of the American Medical Association. 1994; 272 :1672–1677. [ PubMed ] [ Google Scholar ]
- Wechsler H, Dowdall GW, Davenport A, Castillo S. Correlates of college student binge drinking. American Journal of Public Health. 1995; 85 :921–926. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wechsler H, Dowdall GW, Davenport A, Rimm EB. A gender-specific measure of binge drinking among college students. American Journal of Public Health. 1995; 85 :982–985. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wechsler H, Nelson TF. Binge drinking and the American college student: What's five drinks? Psychology of Addictive Behaviors. 2001; 15 :287–291. [ PubMed ] [ Google Scholar ]
- Wechsler H, Nelson TF. Relationship between level of consumption and harms in assessing drink cut-points for alcohol research: Commentary on “Many college freshmen drink at levels far beyond the binge threshold” by White et al. Alcoholism: Clinical and Experimental Research. 2006; 30 :922–927. [ PubMed ] [ Google Scholar ]
- Weingardt KR, Baer JS, Kivlahan DR, Roberts LJ, Miller ET, Marlatt GA. Episodic heavy drinking among college students: Methodological issues and longitudinal perspectives. Psychology of Addictive Behaviors. 1998; 12 :155–167. [ Google Scholar ]
- Weissenborn R, Duka T. Acute alcohol effects on cognitive function in social drinkers: Their relationship to drinking habits. Psychopharmacology. 2003; 165 :306–312. [ PubMed ] [ Google Scholar ]
- Weitzman ER, Nelson TF, Wechsler H. Taking up binge drinking in college: The influences of person, social group, and environment. Journal of Adolescent Health. 2003; 32 :26–35. [ PubMed ] [ Google Scholar ]
- Wezeman FH, Juknelis D, Himes R, Callaci JJ. Vitamin D and ibandronate prevent cancellous bone loss associated with binge alcohol treatment in male rats. Bone. 2007; 41 :639–645. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wiers RW, Stacy AW, Ames SL, Noll JA, Sayette MA, Zack M, et al. Implicit and explicit alcohol-related cognitions. Alcoholism: Clinical and Experimental Research. 2002; 26 :129–137. [ PubMed ] [ Google Scholar ]
- Young JA, Pihl RO. Self-control of the effects of alcohol intoxication. Journal of Studies on Alcohol. 1980; 41 :567–571. [ PubMed ] [ Google Scholar ]
- Zeigler DW, Wang CC, Yoast RA, Dickinson BD, McCaffree MA, Robinowitz CB, et al. The neurocognitive effects of alcohol on adolescents and college students. Preventive Medicine. 2005; 40 :23–32. [ PubMed ] [ Google Scholar ]
- Zou JY, Martinez DB, Neafsey EJ, Collins MA. Binge ethanol-induced brain damage in rats: Effects of inhibitors of nitric oxide synthase. Alcoholism: Clinical and Experimental Research. 1996; 20 :1406–1411. [ PubMed ] [ Google Scholar ]
Determination of regulated perfluoroalkyl substances (PFAS) in drinking water according to Directive 2020/2184/EU
- Environmental Chemistry for A Pollution Free Society
- Published: 12 September 2024
Cite this article

- Javier López-Vázquez 1 ,
- Rosa Montes ORCID: orcid.org/0000-0002-4154-3541 1 ,
- Rosario Rodil 1 ,
- Rafael Cela 2 ,
- José Ángel Martínez-Pontevedra 3 ,
- María Teresa Pena 3 &
- José Benito Quintana 1
1 Altmetric
Perfluoroalkyl substances (PFAS) are chemical compounds that have been widely used in industry and manufacture. Occurrence, together with persistence and recent toxicological effects data, has promoted the regulation of 20 PFAS (carboxylic and sulfonic) acids in drinking water through the recent Directive 2020/2184/EU. This Regulation included PFAS with different carbon chain lengths (from C 4 to C 13 ) and limited the total PFAS concentration (as sum) to a maximum of 0.1 µg/L, for which law-enforcement analytical methods are required. In this work, three different methodologies have been developed and evaluated as regards their performance to determine those 20 PFAS in tap and bottled water, based on on-line and off-line solid-phase extraction (SPE) and direct injection. In all cases, ultra-high pressure liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS) was used as a determination technique. Off-line SPE with Oasis Weak Anion Exchange (WAX) cartridges provided the best results in terms of limits of quantification (LOQ ≤ 0.3 ng/L) and accuracy ( R ≥ 70%) in drinking water samples. On-line SPE and direct injection presented some drawbacks such as background contamination problems and lower accuracies for the least polar compounds. This off-line SPE methodology was then applied to the analysis of 46 drinking water samples (11 commercial bottled samples, 23 Spanish and 12 international tap water samples). Ten PFAS were quantified in such samples at concentrations and detection frequencies ranging from 0.1 to 20.1 ng/L and 2 to 91%, respectively. However, the sum concentration did not surpass the established limit in any sample.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others
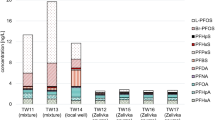
The occurrence of perfluoroalkyl substances (PFAS) in drinking water in the Czech Republic: a pilot study
A single analytical method for the determination of 53 legacy and emerging per- and polyfluoroalkyl substances (PFAS) in aqueous matrices
France-Wide Monitoring of 1,4-Dioxane in Raw and Treated Water: Occurrence and Exposure Via Drinking Water Consumption
Explore related subjects.
- Environmental Chemistry
Data availability
The authors declare that the results from the samples analyzed are compiled in the online repository ZENODO: https://doi.org/10.5281/zenodo.10990801 .
ISO21675/2019 (2019) “ISO 21675/2019 Water quality - determination of perfluoroalkyl and polyfluoroalkyl substances (PFAS) in water - method using solid phase extraction and liquid chromatography-tandem mass spectrometry (LC-MS/MS)”
Al Amin M et al (2020) Recent advances in the analysis of per- and polyfluoroalkyl substances (PFAS)—a review. Environ Technol Innov 19:100879. https://doi.org/10.1016/j.eti.2020.100879
Article Google Scholar
AOAC (2016) “Guidelines for standard method performance requirements” Appendix F. Retrieved from 27 June 2024, from https://www.aoac.org/wp-content/uploads/2019/08/app_f.pdf
Bai X, Son Y (2021) Perfluoroalkyl substances (PFAS) in surface water and sediments from two urban watersheds in Nevada, USA. Sci Total Environ 751:141622–141632. https://doi.org/10.1016/j.scitotenv.2020.141622
Article CAS Google Scholar
Barreca S et al (2018) Online solid-phase extraction LC-MS/MS: a rapid and valid method for the determination of perfluorinated compounds at sub ng·L−1 level in natural water. J Chem 2018:3780825–3780835. https://doi.org/10.1155/2018/3780825
Brandsch T (2022) Determination of PFAS in water according to EU 2020/2184 and DIN 38407–42 using online SPE–LC–MS/MS. The Column 18:8–14
Google Scholar
Castiglioni S et al (2015) Sources and fate of perfluorinated compounds in the aqueous environment and in drinking water of a highly urbanized and industrialized area in Italy. J Hazard Mater 282:51–60. https://doi.org/10.1016/j.jhazmat.2014.06.007
Chow SJ et al (2021) Detection of ultrashort-chain and other per- and polyfluoroalkyl substances (PFAS) in U.S. bottled water. Water Res 201:117292–117303. https://doi.org/10.1016/j.watres.2021.117292
HE Diggs, JM Parker (2009) Chapter 23 - aquatic facilities. Planning and designing research animal facilities. J. R. Hessler and N. D. M. Lehner. London, Academic Press: 323–331
Dufková V et al (2012) Determination of C5–C12 perfluoroalkyl carboxylic acids in river water samples in the Czech Republic by GC–MS after SPE preconcentration. Chemosphere 87:463–469. https://doi.org/10.1016/j.chemosphere.2011.12.029
EC (2013) Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy Text with EEA relevance. Off J Eur Union 226:1–17
EC (2020) Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption. Off J Eur Union 435:1–62
ECHA (2023) “ECHA/NR/23/08. ECHA seeks input on proposed PFAS restriction.” Retrieved 31 July 2023, from https://echa.europa.eu/es/-/echa-seeks-input-on-proposed-pfas-restriction
EEA (2019) Emerging chemical risks in Europe : ‘PFAS’, Publications Office
EFSA et al (2020) Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J 18:e06223–e06614. https://doi.org/10.2903/j.efsa.2020.6223
Gagliano E et al (2020) Removal of poly- and perfluoroalkyl substances (PFAS) from water by adsorption: role of PFAS chain length, effect of organic matter and challenges in adsorbent regeneration. Water Res 171:115381–115412. https://doi.org/10.1016/j.watres.2019.115381
Gao Y et al (2019) Levels, spatial distribution and isomer profiles of perfluoroalkyl acids in soil, groundwater and tap water around a manufactory in China. Chemosphere 227:305–314. https://doi.org/10.1016/j.chemosphere.2019.04.027
Gellrich V et al (2013) Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in mineral water and tap water. J Environ Sci Health Part A 48:129–135. https://doi.org/10.1080/10934529.2013.719431
Glüge J et al (2020) An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ Sci Process Impacts 22:2345–2373. https://doi.org/10.1039/D0EM00291G
González-Barreiro C et al (2006) Method optimization for determination of selected perfluorinated alkylated substances in water samples. Anal Bioanal Chem 386:2123–2132. https://doi.org/10.1007/s00216-006-0902-7
González-Mariño I et al (2019) Plasticizers | Environmental Analysis. In: Worsfold P, Poole C, Townshend A, Miró M (eds) Encyclopedia of Analytical Science (Third Edition). Press, Oxford, Academic, pp 309–317
Gray J et al (2019) Determination of perfluoralkyl substances in drinking and surface waters by LC/MS/MS with online solid-phase extraction: GH11B-1040. AGU fall meeting abstracts. Retrieved 30 October 2023 from https://ui.adsabs.harvard.edu/abs/2019AGUFMGH11B1040G
Gremmel C et al (2017) HPLC–MS/MS methods for the determination of 52 perfluoroalkyl and polyfluoroalkyl substances in aqueous samples. Anal Bioanal Chem 409:1643–1655. https://doi.org/10.1007/s00216-016-0110-z
Gutiérrez-Martín D et al (2023) Chemicals of emerging concern in coastal aquifers: assessment along the land-ocean interface. J Hazard Mater 448:130876. https://doi.org/10.1016/j.jhazmat.2023.130876
Hepburn E et al (2019) Contamination of groundwater with per- and polyfluoroalkyl substances (PFAS) from legacy landfills in an urban re-development precinct. Environ Pollut 248:101–113. https://doi.org/10.1016/j.envpol.2019.02.018
Hernández F et al (2023) Efficient validation strategies in environmental analytical chemistry: a focus on organic micropollutants in water samples. Annu Rev Anal Chem 16:401–428. https://doi.org/10.1146/annurev-anchem-091222-112115
Janda J et al (2019) Robust trace analysis of polar (C2–C8) perfluorinated carboxylic acids by liquid chromatography-tandem mass spectrometry: method development and application to surface water, groundwater and drinking water. Environ Sci Pollut Res 26:7326–7336. https://doi.org/10.1007/s11356-018-1731-x
Kamuf M et al (2020) Reduce PFAS background with the agilent PFC-free HPLC conversion kit. Agilent technologies technical overview. Retrieved 30 October 2023 from https://www.agilent.com/cs/library/technicaloverviews/public/technicaloverview-pfas-background-reduction-pfc-free-hplc-conversion-kit-5994-2291en-agilent.pdf
Kurwadkar S et al (2022) Per- and polyfluoroalkyl substances in water and wastewater: a critical review of their global occurrence and distribution. Sci Total Environ 809:151003–151021. https://doi.org/10.1016/j.scitotenv.2021.151003
Mak YL et al (2009) Perfluorinated compounds in tap water from China and several other countries. Environ Sci Technol 43:4824–4829. https://doi.org/10.1021/es900637a
Montes R et al (2020) Applicability of mixed-mode chromatography for the simultaneous analysis of C1–C18 perfluoroalkylated substances. Anal Bioanal Chem 412:4849–4856. https://doi.org/10.1007/s00216-020-02434-w
Mottaleb MA et al (2021) Direct injection analysis of per and polyfluoroalkyl substances in surface and drinking water by sample filtration and liquid chromatography-tandem mass spectrometry. J Chromatogr A 1653:462426. https://doi.org/10.1016/j.chroma.2021.462426
Mulabagal V et al (2018) A rapid UHPLC-MS/MS method for simultaneous quantitation of 23 perfluoroalkyl substances (PFAS) in estuarine water. Talanta 190:95–102. https://doi.org/10.1016/j.talanta.2018.07.053
OECD (n.d.) “Portal on per and poly fluorinated chemicals.” Retrieved 31 July 2023, from https://www.oecd.org/chemicalsafety/portal-perfluorinated-chemicals/
OECD (2018) “Summary report on the new comprehensive global database of per- and polyfluoroalkyl substances (PFASs)”. https://doi.org/10.1787/1a14ad6c-en
K Organtini et al. (2018) “Large volume direct injection method for the analysis of perfluorinated alkyl substances (PFAS) in environmental water samples in accordance with ASTM 7979–17.” Waters Corporation Application Note 720006329EN
Schwanz TG et al (2016) Perfluoroalkyl substances assessment in drinking waters from Brazil, France and Spain. Sci Total Environ 539:143–152. https://doi.org/10.1016/j.scitotenv.2015.08.034
Tang A et al (2022) Spatiotemporal distribution, partitioning behavior and flux of per- and polyfluoroalkyl substances in surface water and sediment from Poyang Lake, China. Chemosphere 295:133855–133866. https://doi.org/10.1016/j.chemosphere.2022.133855
UCMR3 (2021) “PFAS Sampling in TN.” Retrieved 18/03/2022
ÜnlüEndirlik B et al (2019) Assessment of perfluoroalkyl substances levels in tap and bottled water samples from Turkey. Chemosphere 235:1162–1171. https://doi.org/10.1016/j.chemosphere.2019.06.228
USEPA (2009) Method 537: determination of selected perfluorinated alkyl acids in drinking water by solid phase extraction and liquid chromatography/tandem mass spectrometry (LC/MS/MS). https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NERL&direntryid=198984
USEPA (2019) “Method 533: determination of per- and polyfluoroalkyl substances in drinking water by isotope dilution anion exchange solid phase extraction and liquid chromatography/tandem mass spectrometry.” https://www.epa.gov/sites/default/files/2019-12/documents/method-533-815b19020.pdf
USEPA (2020) “Method 537.1: determination of selected per- and polyfluorinated alkyl substances in drinking water by solid phase extraction and liquid chromatography/tandem mass spectrometry (LC/MS/MS).” https://cfpub.epa.gov/si/si_public_record_Report.cfm?dirEntryId=348508&Lab=CESER
Villaverde-de-Sáa E et al (2015) Solid-phase extraction of perfluoroalkylated compounds from sea water. J Sep Sci 38:1942–1950. https://doi.org/10.1002/jssc.201401453
Wang P et al (2016) Coupled production and emission of short chain perfluoroalkyl acids from a fast developing fluorochemical industry: evidence from yearly and seasonal monitoring in Daling River Basin, China. Environ Pollut 218:1234–1244. https://doi.org/10.1016/j.envpol.2016.08.079
Wolf ST, Reagen WK (2013) Method and validation for the analysis of perfluorinated compounds in water by pre-sampling isotope dilution-direct injection-LC/MS/MS. Anal Methods 5:2444–2454. https://doi.org/10.1039/C3AY26347A
Xu B et al (2021) PFAS and their substitutes in groundwater: occurrence, transformation and remediation. J Hazard Mater 412:125159–125172. https://doi.org/10.1016/j.jhazmat.2021.125159
Yan H et al (2012) Short- and long-chain perfluorinated acids in sewage sludge from Shanghai, China. Chemosphere 88:1300–1305. https://doi.org/10.1016/j.chemosphere.2012.03.105
Zarębska M, Bajkacz S (2023) Poly– and perfluoroalkyl substances (PFAS) - recent advances in the aquatic environment analysis. TrAC Trends Anal Chem 163:117062. https://doi.org/10.1016/j.trac.2023.117062
Download references
Acknowledgements
The authors want to acknowledge Agilent Technologies for lending the LC-MS/MS instrumentation used in this work. We also want to express our gratitude to several colleagues for providing samples: H. Gallard from U. Poitiers (France), T. Reemtsma from UFZ (Germany), S. Castiglioni and N. Salgueiro from Mario Negri Institute (Italy), A. Covaci from U. Antwerp (Netherlands), Y. Picó from CIDE (Spain), L. Bijlsma from UJI (Spain), and M.M. Santos from CIIMAR (Portugal).
The authors acknowledge the funding received by the Spanish Agencia Estatal de Investigación –MCIN/AEI/ 10.13039/501100011033 (ref. PID2020-117686RB-C32 and TED2021-129200B-C41 funded by the European Union through NextGeneration/PRTR funds) and the Xunta de Galicia (ED431C 2021/06). R. Cela acknowledges the Galician Innovation Agency – Xunta de Galicia (001_IN853D-2022).
Author information
Authors and affiliations.
Aquatic One Health Research Center (ARCUS) & Department of Analytical Chemistry, Nutrition and Food Chemistry. R. Constantino Candeira S/N, IIAA Building, Universidade de Santiago de Compostela, 15782, Santiago de Compostela, Spain
Javier López-Vázquez, Rosa Montes, Rosario Rodil & José Benito Quintana
Mestrelab Research Center (CIM), Av. Barcelona 7, 15706, Santiago de Compostela, Spain
Rafael Cela
Applus Norcontrol S.L.U, Carretera NVI Km.582, 15168, Sada, Galicia, Spain
José Ángel Martínez-Pontevedra & María Teresa Pena
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: Rosa Montes, Rosario Rodil, José Benito Quintana.
Methodology: Rosa Montes, Javier López-Vázquez, Rosario Rodil, José Benito Quintana.
Validation: Javier López-Vázquez, Rosa Montes, Rosario Rodil, José Benito Quintana.
Formal analysis: Javier López-Vázquez, Rosa Montes, Rosario Rodil, José Benito Quintana.
Investigation: Javier López-Vázquez.
Resources: Rosario Rodil, José Benito Quintana, Rafael Cela, María Teresa Pena, José Ángel Martínez-Pontevedra.
Writing—original draft: Javier López-Vázquez, Rosa Montes.
Writing—review and editing: All authors.
Visualization: Rosario Rodil, José Benito Quintana.
Supervision: Rosa Montes, Rosario Rodil, José Benito Quintana, María Teresa Pena, José Ángel Martínez-Pontevedra, Rafael Cela.
Project administration: Rosario Rodil, José Benito Quintana.
Funding acquisition: Rosario Rodil, José Benito Quintana, Rafael Cela.
Corresponding author
Correspondence to Rosa Montes .
Ethics declarations
Ethical approval.
The authors declare that there are no personal data in this work that may require ethics committee approval.
Consent to participate
The authors declare that they have consent from the responsible authorities at the institute/organization where the work has been carried out to publish these results.
Consent for publication
The authors declare that there is no individual person’s data in any form in the present work.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Roland Peter Kallenborn
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 515 KB)
Rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
López-Vázquez, J., Montes, R., Rodil, R. et al. Determination of regulated perfluoroalkyl substances (PFAS) in drinking water according to Directive 2020/2184/EU. Environ Sci Pollut Res (2024). https://doi.org/10.1007/s11356-024-34852-z
Download citation
Received : 15 November 2023
Accepted : 22 August 2024
Published : 12 September 2024
DOI : https://doi.org/10.1007/s11356-024-34852-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Perfluoroalkyl carboxylic acids (PFCAs)
- Perfluoroalkyl sulfonic acids (PFSAs)
- Solid-phase extraction (SPE)
- Water quality
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
These research findings can help shape the efforts of communities to reduce the negative consequences of alcohol consumption, assist health practitioners in advising consumers, and help individuals make informed decisions about drinking.
The main purpose of this review article is to enable any person reading this article to get a comprehensive insight into the effects of alcohol on the various systems of the human body, and for the same, many recognized research articles published in numerous well-acknowledged journals across the globe are reviewed.
This systematic review and meta-analysis evaluates the association between daily alcohol intake and risk of all-cause mortality.
Alcohol use disorder is characterized by loss of control over alcohol drinking that is accompanied by changes in brain regions related to the execution of motivated behaviors and to the control of stress and emotionality (e.g., the midbrain, the limbic system, the prefrontal cortex, and the amygdala).
FindingsThis cohort study in 135 103 older drinkers found that even low-risk drinking was associated with higher mortality among older adults with health-related or socioeconomic risk factors. Wine preference and drinking only with meals were associated with attenuating the excess mortality associated with alcohol consumption.
In this prospective longitudinal study of alcohol consumption and patterns in heavy drinking young adults, significant reductions in alcohol use quantity, frequency and problems were observed from ...
No level of alcohol consumption is safe for our health. The risks and harms associated with drinking alcohol have been systematically evaluated over the years and are well documented. The World Health Organization has now published a statement in The Lancet Public Health: when it comes to alcohol consumption, there is no safe amount that does ...
These expectancies were also predictive of higher binge drinking in adolescents but not adults, highlighting the importance of future research into age differences in alcohol-related cognitions ...
As Demant (2009) notes: 'In research on alcohol and youth, concepts like drinking pressure, excuse value and peer pressure often have a tone of "invisible agency", which ascribes power to a collective that works behind the backs of drinking [or non-drinking] teenagers' (p. 29).
This WHO fact sheet on alcohol provides key facts, who is at risk, ways to reduce the burden, and WHO;s response.
Heavy drinking and alcohol use disorder (AUD) are major public health problems. Practitioners not specializing in alcohol treatment are often unaware of the guidelines for preventing, identifying, and treating heavy drinking and AUD. However, a consensus exists that clinically useful and valuable tools are available to address these issues.
Recent research makes it clear that any amount of drinking can be detrimental. Here's why you may want to cut down on your consumption beyond Dry January.
Despite legal sanctions in place, people continue to drive after drinking alcohol; in fact, it was reported that drivers impaired by alcohol were responsible for 147 million instances of driving under the influence in 2018 (Centers for Disease Control and Prevention, 2020).
Psychologists' research is pinpointing who is most at risk for drinking problems in college and developing more targeted, evidence-based interventions.
The past 50 years of research supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) have resulted in an accumulation of invaluable data to address the multifaceted problems surrounding underage drinking.
Published article by Ruth C. Engs, "Why drinking age should be lowered: An opinion based on research"
What Is Binge Drinking? The National Institute on Alcohol Abuse and Alcoholism (NIAAA) defines binge drinking as a pattern of drinking alcohol that brings blood alcohol concentration (BAC) to 0.08%—or 0.08 grams of alcohol per deciliter—or more. This typically happens if a woman has four or more drinks, or a man has five or more drinks, within about 2 hours.1 Research shows that fewer ...
Binge drinking is an increasingly important topic in alcohol research, but the field lacks empirical cohesion and definitional precision. The present review summarizes findings and viewpoints from the scientific binge-drinking literature. Epidemiological ...
Perfluoroalkyl substances (PFAS) are chemical compounds that have been widely used in industry and manufacture. Occurrence, together with persistence and recent toxicological effects data, has promoted the regulation of 20 PFAS (carboxylic and sulfonic) acids in drinking water through the recent Directive 2020/2184/EU. This Regulation included PFAS with different carbon chain lengths (from C4 ...