- Search by keyword
- Search by citation
Page 1 of 33

CCL5 is essential for axonogenesis and neuronal restoration after brain injury
Traumatic brain injury (TBI) causes axon tearing and synapse degradation, resulting in multiple neurological dysfunctions and exacerbation of early neurodegeneration; the repair of axonal and synaptic structur...
- View Full Text

Decreased plasma gelsolin fosters a fibrotic tumor microenvironment and promotes chemoradiotherapy resistance in esophageal squamous cell carcinoma
Stromal fibrosis is highly associated with therapeutic resistance and poor survival in esophageal squamous cell carcinoma (ESCC) patients. Low expression of plasma gelsolin (pGSN), a serum abundant protein, ha...
Current landscape of mRNA technologies and delivery systems for new modality therapeutics
Realizing the immense clinical potential of mRNA-based drugs will require continued development of methods to safely deliver the bioactive agents with high efficiency and without triggering side effects. In th...
Tudor-SN exacerbates pathological vascular remodeling by promoting the polyubiquitination of PTEN via NEDD4-1
Dysregulation of vascular homeostasis can induce cardiovascular diseases and increase global mortality rates. Although lineage tracing studies have confirmed the pivotal role of modulated vascular smooth muscl...
Neuroprotective effects of intranasal extracellular vesicles from human platelet concentrates supernatants in traumatic brain injury and Parkinson’s disease models
The burgeoning field of regenerative medicine has significantly advanced with recent findings on biotherapies using human platelet lysates (HPLs), derived from clinical-grade platelet concentrates (PCs), for t...

Dengue NS1 interaction with lipids alters its pathogenic effects on monocyte derived macrophages
While dengue NS1 antigen has been shown to be associated with disease pathogenesis in some studies, it has not been linked in other studies, with the reasons remaining unclear. NS1 antigen levels in acute deng...
Extracellular vesicle therapy in neurological disorders
Extracellular vesicles (EVs) are vital for cell-to-cell communication, transferring proteins, lipids, and nucleic acids in various physiological and pathological processes. They play crucial roles in immune mo...

Machine learning enabled classification of lung cancer cell lines co-cultured with fibroblasts with lightweight convolutional neural network for initial diagnosis
Identification of lung cancer subtypes is critical for successful treatment in patients, especially those in advanced stages. Many advanced and personal treatments require knowledge of specific mutations, as w...
Beyond glycan barriers: non-cognate ligands and protein mimicry approaches to elicit broadly neutralizing antibodies for HIV-1
Human immunodeficiency virus type 1 (HIV-1) vaccine immunogens capable of inducing broadly neutralizing antibodies (bNAbs) remain obscure. HIV-1 evades immune responses through enormous diversity and hides its...

Plexin C1 influences immune response to intracellular LPS and survival in murine sepsis
Intracellular sensing of lipopolysaccharide (LPS) is essential for the immune response against gram-negative bacteria and results in activation of caspase-11 and pyroptotic cell death with fatal consequences i...
EpCAM-targeted betulinic acid analogue nanotherapy improves therapeutic efficacy and induces anti-tumorigenic immune response in colorectal cancer tumor microenvironment
Betulinic acid (BA) has been well investigated for its antiproliferative and mitochondrial pathway-mediated apoptosis-inducing effects on various cancers. However, its poor solubility and off-target activity h...

Low-level HIV-1 viremia affects T-cell activation and senescence in long-term treated adults in the INSTI era
Around 10% of people with HIV (PWH) exhibit a low-level viremia (LLV) under antiretroviral therapy (ART). However, its origin and clinical significance are largely unknown, particularly at viremias between 50 ...
Gene therapy for ultrarare diseases: a geneticist’s perspective
Gene therapy has made considerable strides in recent years. More than 4000 protein-coding genes have been implicated in more than 6000 genetic diseases; next-generation sequencing has dramatically revolutioniz...
Targeted nanotherapeutics for the treatment of Helicobacter pylori infection
Helicobacter pylori infection is involved in gastric diseases such as peptic ulcer and adenocarcinoma. Approved antibiotherapies still fail in 10 to 40% of the infected patients and, in this scenario, targeted na...
Variants of human DECTIN-1 rs16910526 are linked to differential reactive oxygen species production and susceptibility to tuberculosis
Dectin-1 is a transmembrane receptor that plays a pivotal role in recognising fungi and Mycobacterium tuberculosis (Mtb) . A specific variant, DECTIN-1 rs16910526, results in a truncated receptor that disrupts mem...

Retraction Note: Inhibitors of apoptosis proteins in experimental benign prostatic hyperplasia: effects of Serenoa repens , selenium and lycopene
Development of novel antimicrobials with engineered endolysin lysecd7-smap to combat gram-negative bacterial infections.
Among the non-traditional antibacterial agents in development, only a few targets critical Gram-negative bacteria such as carbapenem-resistant Pseudomonas aeruginosa , Acinetobacter baumannii or cephalosporin-resi...
BUB1B monoallelic germline variants contribute to prostate cancer predisposition by triggering chromosomal instability
Prostate cancer (PrCa) is the most frequently diagnosed cancer in men. Variants in known moderate- to high-penetrance genes explain less than 5% of the cases arising at early-onset (< 56 years) and/or with fam...
Enteroviruses: epidemic potential, challenges and opportunities with vaccines
Enteroviruses (EVs) are the most prevalent viruses in humans. EVs can cause a range of acute symptoms, from mild common colds to severe systemic infections such as meningitis, myocarditis, and flaccid paralysi...
Intracellular domain of epithelial cell adhesion molecule induces Wnt receptor transcription to promote colorectal cancer progression
Epithelial cell adhesion molecule (EpCAM) has been widely studied as a tumor antigen due to its expression in varieties of solid tumors. Moreover, the glycoprotein contributes to critical cancer-associated cel...
Unraveling the differential mechanisms of revascularization promoted by MSCs & ECFCs from adipose tissue or umbilical cord in a murine model of critical limb-threatening ischemia
Critical limb-threatening ischemia (CLTI) constitutes the most severe manifestation of peripheral artery disease, usually induced by atherosclerosis. CLTI patients suffer from high risk of amputation of the lo...
A glimpse into viral warfare: decoding the intriguing role of highly pathogenic coronavirus proteins in apoptosis regulation
Coronaviruses employ various strategies for survival, among which the activation of endogenous or exogenous apoptosis stands out, with viral proteins playing a pivotal role. Notably, highly pathogenic coronavi...
CPEB2-activated axonal translation of VGLUT2 mRNA promotes glutamatergic transmission and presynaptic plasticity
Local translation at synapses is important for rapidly remodeling the synaptic proteome to sustain long-term plasticity and memory. While the regulatory mechanisms underlying memory-associated local translatio...
Dual inhibition of SUMOylation and MEK conquers MYC-expressing KRAS -mutant cancers by accumulating DNA damage
KRAS mutations frequently occur in cancers, particularly pancreatic ductal adenocarcinoma, colorectal cancer, and non-small cell lung cancer. Although KRAS G12C inhibitors have recently been approved, effective pr...
Exosomes: a review of biologic function, diagnostic and targeted therapy applications, and clinical trials
Exosomes are extracellular vesicles generated by all cells and they carry nucleic acids, proteins, lipids, and metabolites. They mediate the exchange of substances between cells,thereby affecting biological pr...
Cholestasis-induced phenotypic transformation of neutrophils contributes to immune escape of colorectal cancer liver metastasis
Cholestasis is a common yet severe complication that occurs during the advancement of liver metastasis. However, how cholestasis impacts the development, treatment, and tumor microenvironment (TME) of liver me...

Enterovirus-A71 exploits RAB11 to recruit chaperones for virus morphogenesis
Enterovirus 71 (EV-A71) causes Hand, Foot and Mouth Disease (HFMD) in children and has been associated with neurological complications. The molecular mechanisms involved in EV-A71 pathogenesis have remained el...
The double whammy of ER-retention and dominant-negative effects in numerous autosomal dominant diseases: significance in disease mechanisms and therapy
The endoplasmic reticulum (ER) employs stringent quality control mechanisms to ensure the integrity of protein folding, allowing only properly folded, processed and assembled proteins to exit the ER and reach ...
Beyond traditional translation: ncRNA derived peptides as modulators of tumor behaviors
Within the intricate tapestry of molecular research, noncoding RNAs (ncRNAs) were historically overshadowed by a pervasive presumption of their inability to encode proteins or peptides. However, groundbreaking...
Adipocyte pyroptosis occurs in omental tumor microenvironment and is associated with chemoresistance of ovarian cancer
Ovarian carcinoma (OC) is a fatal malignancy, with most patients experiencing recurrence and resistance to chemotherapy. In contrast to hematogenous metastasizing tumors, ovarian cancer cells disseminate withi...
Correction: Excess glucose alone depress young mesenchymal stromal/stem cell osteogenesis and mitochondria activity within hours/days via NAD + / SIRT1 axis
The original article was published in Journal of Biomedical Science 2024 31 :49
The glycosylation deficiency of flavivirus NS1 attenuates virus replication through interfering with the formation of viral replication compartments
Flavivirus is a challenge all over the world. The replication of flavivirus takes place within membranous replication compartments (RCs) derived from endoplasmic reticulum (ER). Flavivirus NS1 proteins have be...
Osteosarcoma in a ceRNET perspective
Osteosarcoma (OS) is the most prevalent and fatal type of bone tumor. It is characterized by great heterogeneity of genomic aberrations, mutated genes, and cell types contribution, making therapy and patients ...
Immunoglobulin and T cell receptor repertoire changes induced by a prototype vaccine against Chagas disease in naïve rhesus macaques
A vaccine against Trypanosoma cruzi , the agent of Chagas disease, would be an excellent additional tool for disease control. A recombinant vaccine based on Tc24 and TSA1 parasite antigens was found to be safe and...
Revolution in sepsis: a symptoms-based to a systems-based approach?
Severe infection and sepsis are medical emergencies. High morbidity and mortality are linked to CNS dysfunction, excessive inflammation, immune compromise, coagulopathy and multiple organ dysfunction. Males ap...
Development of a highly effective combination monoclonal antibody therapy against Herpes simplex virus
Infections with Herpes simplex virus (HSV)-1 or -2 usually present as mild chronic recurrent disease, however in rare cases can result in life-threatening conditions with a large spectrum of pathology. Monoclo...
USP9X-mediated REV1 deubiquitination promotes lung cancer radioresistance via the action of REV1 as a Rad18 molecular scaffold for cystathionine γ-lyase
Radioresistance is a key clinical constraint on the efficacy of radiotherapy in lung cancer patients. REV1 DNA directed polymerase (REV1) plays an important role in repairing DNA damage and maintaining genomic...
Tumor necrosis factor-inducible gene 6 protein and its derived peptide ameliorate liver fibrosis by repressing CD44 activation in mice with alcohol-related liver disease
Alcohol-related liver disease (ALD) is a major health concern worldwide, but effective therapeutics for ALD are still lacking. Tumor necrosis factor-inducible gene 6 protein (TSG-6), a cytokine released from m...
Characterization of the genetic variation and evolutionary divergence of the CLEC18 family
The C-type lectin family 18 (CLEC18) with lipid and glycan binding capabilities is important to metabolic regulation and innate immune responses against viral infection. However, human CLEC18 comprises three p...
Pivotal functions and impact of long con-coding RNAs on cellular processes and genome integrity
Recent advances in uncovering the mysteries of the human genome suggest that long non-coding RNAs (lncRNAs) are important regulatory components. Although lncRNAs are known to affect gene transcription, their m...
Somatic PDGFRB activating variants promote smooth muscle cell phenotype modulation in intracranial fusiform aneurysm
The fusiform aneurysm is a nonsaccular dilatation affecting the entire vessel wall over a short distance. Although PDGFRB somatic variants have been identified in fusiform intracranial aneurysms, the molecular...
A G-quadruplex-binding platinum complex induces cancer mitochondrial dysfunction through dual-targeting mitochondrial and nuclear G4 enriched genome
G-quadruplex DNA (G4) is a non-canonical structure forming in guanine-rich regions, which play a vital role in cancer biology and are now being acknowledged in both nuclear and mitochondrial (mt) genome. Howev...

Excess glucose alone depress young mesenchymal stromal/stem cell osteogenesis and mitochondria activity within hours/days via NAD + /SIRT1 axis
The impact of global overconsumption of simple sugars on bone health, which peaks in adolescence/early adulthood and correlates with osteoporosis (OP) and fracture risk decades, is unclear. Mesenchymal stromal...
The Correction to this article has been published in Journal of Biomedical Science 2024 31 :61
Contribution of extracellular vesicles for the pathogenesis of retinal diseases: shedding light on blood-retinal barrier dysfunction
Retinal degenerative diseases, including diabetic retinopathy (DR) and age-related macular degeneration (AMD), loom as threats to vision, causing detrimental effects on the structure and function of the retina...
Exploiting urine-derived induced pluripotent stem cells for advancing precision medicine in cell therapy, disease modeling, and drug testing
The field of regenerative medicine has witnessed remarkable advancements with the emergence of induced pluripotent stem cells (iPSCs) derived from a variety of sources. Among these, urine-derived induced pluri...
Targeting cathepsin S promotes activation of OLF1-BDNF/TrkB axis to enhance cognitive function
Cathepsin S (CTSS) is a cysteine protease that played diverse roles in immunity, tumor metastasis, aging and other pathological alterations. At the cellular level, increased CTSS levels have been associated wi...
Campylobacter jejuni virulence factors: update on emerging issues and trends
Campylobacter jejuni is a very common cause of gastroenteritis, and is frequently transmitted to humans through contaminated food products or water. Importantly, C. jejuni infections have a range of short- and l...
Membrane lipid remodeling eradicates Helicobacter pylori by manipulating the cholesteryl 6'-acylglucoside biosynthesis
Helicobacter pylori , the main cause of various gastric diseases, infects approximately half of the human population. This pathogen is auxotrophic for cholesterol which it converts to various cholesteryl α-glucosi...

Dengue virus pathogenesis and host molecular machineries
Dengue viruses (DENV) are positive-stranded RNA viruses belonging to the Flaviviridae family. DENV is the causative agent of dengue, the most rapidly spreading viral disease transmitted by mosquitoes. Each yea...
T cell expressions of aberrant gene signatures and Co-inhibitory receptors (Co-IRs) as predictors of renal damage and lupus disease activity
Systemic lupus erythematosus (SLE) is distinguished by an extensive range of clinical heterogeneity with unpredictable disease flares and organ damage. This research investigates the potential of aberrant sign...
- Editorial Board
- Manuscript editing services
- Instructions for Editors
- Sign up for article alerts and news from this journal

Journal of Biomedical Science is supported by the National Science and Technology Council (NSTC) , Taiwan.
Annual Journal Metrics
Citation Impact 2023 Journal Impact Factor: 9.0 5-year Journal Impact Factor: 10.7 Source Normalized Impact per Paper (SNIP): 2.014 SCImago Journal Rank (SJR): 2.606
Speed 2023 Submission to first editorial decision (median days): 14 Submission to acceptance (median days): 107
Usage 2023 Downloads: 1,698,723 Altmetric mentions: 3,813
- More about our metrics
Journal of Biomedical Science
ISSN: 1423-0127
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Ask the publishers to restore access to 500,000+ books.
Send me an email reminder
By submitting, you agree to receive donor-related emails from the Internet Archive. Your privacy is important to us. We do not sell or trade your information with anyone.
Internet Archive Audio

- Grateful Dead
- Old Time Radio
- 78 RPMs and Cylinder Recordings
- Audio Books & Poetry
- Computers, Technology and Science
- Music, Arts & Culture
- News & Public Affairs
- Spirituality & Religion
- Radio News Archive

- Flickr Commons
- Occupy Wall Street Flickr
- NASA Images
- Solar System Collection
- Ames Research Center

- All Software
- Old School Emulation
- MS-DOS Games
- Historical Software
- Classic PC Games
- Software Library
- Kodi Archive and Support File
- Vintage Software
- CD-ROM Software
- CD-ROM Software Library
- Software Sites
- Tucows Software Library
- Shareware CD-ROMs
- Software Capsules Compilation
- CD-ROM Images
- ZX Spectrum
- DOOM Level CD

- Smithsonian Libraries
- FEDLINK (US)
- Lincoln Collection
- American Libraries
- Canadian Libraries
- Universal Library
- Project Gutenberg
- Children's Library
- Biodiversity Heritage Library
- Books by Language
- Additional Collections

- Prelinger Archives
- Democracy Now!
- Occupy Wall Street
- TV NSA Clip Library
- Animation & Cartoons
- Arts & Music
- Computers & Technology
- Cultural & Academic Films
- Ephemeral Films
- Sports Videos
- Videogame Videos
- Youth Media
Search the history of over 866 billion web pages on the Internet.
Mobile Apps
- Wayback Machine (iOS)
- Wayback Machine (Android)
Browser Extensions
Archive-it subscription.
- Explore the Collections
- Build Collections
Save Page Now
Capture a web page as it appears now for use as a trusted citation in the future.
Please enter a valid web address
- Donate Donate icon An illustration of a heart shape
Essentials of writing biomedical research papers
Bookreader item preview, share or embed this item, flag this item for.
- Graphic Violence
- Explicit Sexual Content
- Hate Speech
- Misinformation/Disinformation
- Marketing/Phishing/Advertising
- Misleading/Inaccurate/Missing Metadata
![[WorldCat (this item)] [WorldCat (this item)]](https://archive.org/images/worldcat-small.png)
plus-circle Add Review comment Reviews
87 Previews
Better World Books
DOWNLOAD OPTIONS
No suitable files to display here.
PDF access not available for this item.
IN COLLECTIONS
Uploaded by station64.cebu on April 1, 2023
SIMILAR ITEMS (based on metadata)
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
PubMed Central (PMC) Home Page
PubMed Central ® (PMC) is a free full-text archive of biomedical and life sciences journal literature at the U.S. National Institutes of Health's National Library of Medicine (NIH/NLM)
Discover a digital archive of scholarly articles, spanning centuries of scientific research.
Learn how to find and read articles of interest to you.
Collections
Browse the PMC Journal List or learn about some of PMC's unique collections.
For Authors
Navigate the PMC submission methods to comply with a funder mandate, expand access, and ensure preservation.
For Publishers
Learn about deposit options for journals and publishers and the PMC selection process.
For Developers
Find tools for bulk download, text mining, and other machine analysis.
10.2 MILLION articles are archived in PMC.
Content provided in part by:, full participation journals.
Journals deposit the complete contents of each issue or volume.

NIH Portfolio Journals
Journals deposit all NIH-funded articles as defined by the NIH Public Access Policy.
Selective Deposit Programs
Publisher deposits a subset of articles from a collection of journals.
Sept. 16, 2024
Pmc website update coming october 15.
On October 15, 2024, PMC will transition to a new website. This update, available for preview since March 2024 for user test…
March 21, 2024
Preview upcoming improvements to pmc.
We are pleased to announce the availability of a preview of improvements planned for the PMC website. These…

We are pleased to announce the availability of a preview of improvements planned for the PMC website. These improvements will become the default in October 2024.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Download Free PDF
Essentials of writing biomedical research papers

Chapter 5: Materials and Methods
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
- ORGANIZATION
- SUMMARY OF GUIDELINES FOR THE MATERIALS AND METHODS SECTION
- EXERCISE 5.1: A CLEARLY WRITTEN METHODS SECTION
- EXERCISE 5.2: CONTENT AND ORGANIZATION IN THE METHODS SECTION
- Full Chapter
- Supplementary Content
For hypothesis-testing papers, the function of the Materials and Methods section (often referred to as the Methods section) is to tell the reader what experiments you did to answer the question posed in the Introduction. Similarly, for descriptive studies, the Methods section tells what experiments you did to obtain the message stated in the Introduction. For methods papers, the Methods section has two functions: it describes the new method in complete detail and also tells what experiments you did to test the new method. For all types of paper, the Methods section should include sufficient detail and references to permit a trained scientist to evaluate your work fully or to repeat the experiments exactly as you did them.
Hypothesis-Testing and Descriptive Papers
We saw that the first step in the story line of a hypothesis-testing or a descriptive paper is presented in the Introduction. This first step is either the question being asked or the structure being described. In either case, the second step in the story line is an overview of the experiments you did. This overview of the experiments gives the strategy of the experiments, the plan that connects the methods to each other and to the question or the message.
Where the overview of the experiments is presented depends on the type of research:
| Placement of the Overview of the Experiments | Example | |
|---|---|---|
| Descriptive research | In the experimental approach at the end of the Introduction | Structure-function studies |
| Hypothesis-testing research in which one experiment determines what the next experiment will be | In the experimental approach at the end of the Introduction (and then threaded through the Results section) (see , Results) | Some biochemistry studies |
| Hypothesis-testing research in which all experiments are designed in advance | In the Study Design subsection of the Materials and Methods section | Physiology studies Clinical studies Some biochemistry studies |
Methods Papers
For a Methods paper, the first step in the story line is a statement that you are presenting a new or improved material, method, or apparatus. The second step in the story line has two parts: a complete description of the new method, material, or apparatus; and a description of how this new method, material, or apparatus was tested. These two steps are described in the Methods section.
In this chapter, we will consider only Methods sections for hypothesis-testing papers.
Get Free Access Through Your Institution
Download the Access App: iOS | Android
Pop-up div Successfully Displayed
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
- Corpus ID: 30091851
Essentials of writing biomedical research papers , second edition
- Published 2001
- Biology, Medicine
3 Citations
How to write your first research paper, years before the use of checklists became a manifesto for saving lives, the story line for a hypothesis testing paper :, related papers.
Showing 1 through 3 of 0 Related Papers
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Perspective
- Published: 16 January 2023
The next generation of evidence-based medicine
- Vivek Subbiah ORCID: orcid.org/0000-0002-6064-6837 1 , 2 , 3
Nature Medicine volume 29 , pages 49–58 ( 2023 ) Cite this article
164k Accesses
188 Citations
787 Altmetric
Metrics details
- Adaptive clinical trial
- Drug development
- Health policy
Recently, advances in wearable technologies, data science and machine learning have begun to transform evidence-based medicine, offering a tantalizing glimpse into a future of next-generation ‘deep’ medicine. Despite stunning advances in basic science and technology, clinical translations in major areas of medicine are lagging. While the COVID-19 pandemic exposed inherent systemic limitations of the clinical trial landscape, it also spurred some positive changes, including new trial designs and a shift toward a more patient-centric and intuitive evidence-generation system. In this Perspective, I share my heuristic vision of the future of clinical trials and evidence-based medicine.
Similar content being viewed by others
Looking back and thinking forwards — 15 years of cardiology and cardiovascular research

Developing robust benchmarks for driving forward AI innovation in healthcare
Causal inference and counterfactual prediction in machine learning for actionable healthcare
The last 30 years have witnessed breathtaking, unparalleled advancements in scientific research—from a better understanding of the pathophysiology of basic disease processes and unraveling the cellular machinery at atomic resolution to developing therapies that alter the course and outcome of diseases in all areas of medicine. Moreover, exponential gains in genomics, immunology, proteomics, metabolomics, gut microbiomes, epigenetics and virology in parallel with big data science, computational biology and artificial intelligence (AI) have propelled these advances. In addition, the dawn of CRISPR–Cas9 technologies has opened a tantalizing array of opportunities in personalized medicine.
Despite these advances, their rapid translation from bench to bedside is lagging in most areas of medicine and clinical research remains outpaced. The drug development and clinical trial landscape continues to be expensive for all stakeholders, with a very high failure rate. In particular, the attrition rate for early-stage developmental therapeutics is quite high, as more than two-thirds of compounds succumb in the ‘valley of death’ between bench and bedside 1 , 2 . To bring a drug successfully through all phases of drug development into the clinic costs more than 1.5–2.5 billion dollars (refs. 3 , 4 ). This, combined with the inherent inefficiencies and deficiencies that plague the healthcare system, is leading to a crisis in clinical research. Therefore, innovative strategies are needed to engage patients and generate the necessary evidence to propel new advances into the clinic, so that they may improve public health. To achieve this, traditional clinical research models should make way for avant-garde ideas and trial designs.
Before the COVID-19 pandemic, the conduct of clinical research had remained almost unchanged for 30 years and some of the trial conduct norms and rules, although archaic, were unquestioned. The pandemic exposed many of the inherent systemic limitations in the conduct of trials 5 and forced the clinical trial research enterprise to reevaluate all processes—it has therefore disrupted, catalyzed and accelerated innovation in this domain 6 , 7 . The lessons learned should help researchers to design and implement next-generation ‘patient-centric’ clinical trials.
Chronic diseases continue to impact millions of lives and cause major financial strain to society 8 , but research is hampered by the fact that most of the data reside in data silos. The subspecialization of the clinical profession has led to silos within and among specialties; every major disease area seems to work completely independently. However, the best clinical care is provided in a multidisciplinary manner with all relevant information available and accessible. Better clinical research should harness the knowledge gained from each of the specialties to achieve a collaborative model enabling multidisciplinary, high-quality care and continued innovation in medicine. Because many disciplines in medicine view the same diseases differently—for example, infectious disease specialists view COVID-19 as a viral disease while cardiology experts view it as an inflammatory one—cross-discipline approaches will need to respect the approaches of other disciplines. Although a single model may not be appropriate for all diseases, cross-disciplinary collaboration will make the system more efficient to generate the best evidence.
Over the next decade, the application of machine learning, deep neural networks and multimodal biomedical AI is poised to reinvigorate clinical research from all angles, including drug discovery, image interpretation, streamlining electronic health records, improving workflow and, over time, advancing public health (Fig. 1 ). In addition, innovations in wearables, sensor technology and Internet of Medical Things (IoMT) architectures offer many opportunities (and challenges) to acquire data 9 . In this Perspective, I share my heuristic vision of the future of clinical trials and evidence generation and deliberate on the main areas that need improvement in the domains of clinical trial design, clinical trial conduct and evidence generation.
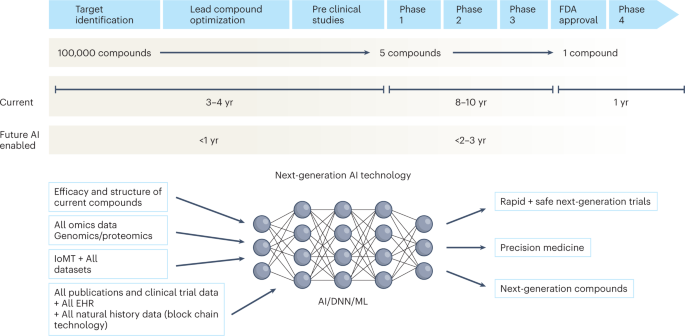
The figure represents the timeline from drug discovery to first-in-human phase 1 trials and ultimately FDA approval. Phase 4 studies occur after FDA approval and can go on for several years. There is an urgent need to reinvigorate clinical trials through drug discovery, interpreting imaging, streamlining electronic health records, and improving workflow, over time advancing public health. AI can aid in many of these aspects in all stages of drug development. DNN, deep neural network; EHR, electronic health records; IoMT, internet of medical things; ML, machine learning.
Clinical trial design
Trial design is one of the most important steps in clinical research—better protocol designs lead to better clinical trial conduct and faster ‘go/no-go’ decisions. Moreover, losses from poorly designed, failed trials are not only financial but also societal.
Challenges with randomized controlled trials
Randomized controlled trials (RCTs) have been the gold standard for evidence generation across all areas of medicine, as they allow unbiased estimates of treatment effect without confounders. Ideally, every medical treatment or intervention should be tested via a well-powered and well-controlled RCT. However, conducting RCTs is not always feasible owing to challenges in generating evidence in a timely manner, cost, design on narrow populations precluding generalizability, ethical barriers and the time taken to conduct these trials. By the time they are completed and published, RCTs become quickly outdated and, in some cases, irrelevant to the current context. In the field of cardiology alone, 30,000 RCTs have not been completed owing to recruitment challenges 10 . Moreover, trials are being designed in isolation and within silos, with many clinical questions remaining unanswered. Thus, traditional trial design paradigms must adapt to contemporary rapid advances in genomics, immunology and precision medicine 11 .
Progress in clinical trial design
High-quality evidence is needed for clinical practice, which has traditionally been achieved with RCTs 12 . In the last decade, substantial progress has been made in the design, conduct and implementation of ‘master’ protocols (overarching protocols that apply to several substudies), which has led to many practice changes that have substantially improved the stagnation of RCTs. Moreover, master protocols may involve parallel interventional studies in a single disease or multiple diseases defined by a biomarker or disease entity 12 . Four different classes of studies are included under the master protocols—the umbrella study, basket study, platform study and master observational trial (MOT) (Fig. 2 ). Each of these is a unique trial design that can include independent arms with control interventions and may be analyzed individually and/or collectively, with added flexibility 13 , 14 . The field of oncology has led these efforts more so than any other field, owing to advances in genomics (for identifying molecular alterations), discovery of therapeutics and rapid clinical translation, thus ushering in the precision oncology era.
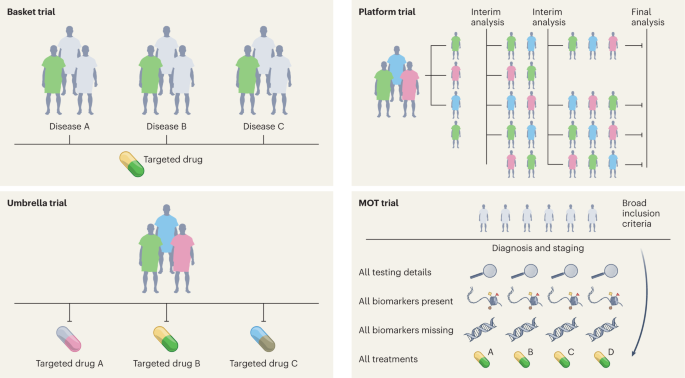
Four different classes of studies are included under the master protocols—the basket study, umbrella study, platform study and MOT.
Umbrella study
Umbrella trials are study designs that evaluate multiple targeted therapies for the same disease entity, stratified by molecular alteration. Examples include the I-SPY (Investigation of Serial Studies to Predict Your Therapeutic Response With Imaging And Molecular Analysis) breast cancer trial and Lung-MAP (Lung Cancer Master Protocol) 15 , 16 .
Basket (or bucket) trial
Basket trials are tissue-agnostic or histology-independent studies where targeted therapy is evaluated on multiple disease types that all harbor the same underlying molecular aberration. For instance, the VE-Basket study (in which VE denotes vemurafenib) 17 , Rare Oncology Agnostic Research (ROAR) study 18 , ARROW trial 19 and LIBRETTO-001 trials 20 , 21 have led to several drug approvals in specific biomarker-driven populations in a histology-dependent and histology-independent manner.
Platform study
These are multi-arm, multistage study designs that compare several intervention groups with a common control group in the context of the same master protocol. Additionally, they can be perpetual/immortal study designs (with no defined end date) and are more efficient than traditional trials on account of the shared control arm, which ensures that a greater proportion of patients are enrolled in the interventional/experimental arms than in the control arm. The Randomised Evaluation of COVID-19 Therapy (RECOVERY) Platform Study is a prominent example; this practice-changing trial established dexamethasone as an effective treatment for COVID-19 (ref. 22 ) and also showed that hydroxychloroquine was ineffective. Platform studies are flexible by design and do not necessarily need to have a shared control arm; the main idea is that intervention arms may be added to an ongoing trial, for example, as in the The UK Plasma Based Molecular Profiling of Advanced Breast Cancer to Inform Therapeutic CHoices (plasmaMATCH) platform trial 23 . Although the aforementioned trials were designed in the context of drug development in oncology and infectious diseases, the scope of platform trials could be leveraged in other diverse areas such as clinical psychology and neurology 24 . Such trials could also be used for digital mental health interventions and could be readily implemented in resource-constrained settings 24 .
The MOT is a prospective, observational study design that broadly accepts patients independently of biomarker signature and collects comprehensive data on each participant 14 , 25 . The MOT is a combination of the master interventional trial and prospective observational trial designs and attempts to hybridize the power of biomarker-based master interventional protocols with the breadth of real-world data (RWD) 14 , 25 . This approach could be well suited to collect prospective RWD across many specialties; the Registry of Oncology Outcomes Associated with Testing and Treatment (ROOT) MOT is one example 14 .
Development of biomarkers and defining endpoints
Biomarker development has facilitated progress in clinical trial design, with unprecedented advances in genomics and immunology leading to several approvals for biomarker-based targeted therapies and immunotherapy in the last decade. In fact, human genetics evidence provided support for more than two-thirds of the drug approvals in 2021 (ref. 26 ). The fields of oncology and genetics have benefited immensely from these advances, but fields such as cardiology, nephrology and pulmonology are still lagging in biomarker-based drug approvals.
To fast-track drug development and clinical trials in every major disease, we will need to define biomarkers (whether clinical, pathological or physiological) and their context of use for every disease process and delineate clear endpoints for studies 27 . Biomarkers can be diagnostic, prognostic or predictive and can inform early drug development, dose selection and trial design. In addition, biomarkers can help to fast-track basic science and drug discovery—all with the eventual goal of improving patient health 28 . However, the level of evidence for a biomarker largely depends on the context of use.
In addition to biomarkers, every field needs to define areas of top priority for research and identify the most relevant endpoints to answer priority research questions. Endpoints are measures of health and/or disease and serve different purposes depending on the phase of the trial 28 , 29 . Beyond clinical and regulatory endpoints, patient-reported outcomes and digital endpoints are also rapidly emerging.
Digital endpoints
Digital endpoints are sensor-generated data collected outside the clinical environment in the context of patients’ routine living—such as using smartphone microphones to monitor cognitive decline in people with Alzheimer’s disease or smartwatch monitors to evaluate drug effect in people with sickle-cell anemia 29 . This is an area of considerable excitement in medicine as it could permit more realistic real-world tracking of the patient experience. Moreover, with the increase in decentralized trial conduct across many specialties, remote monitoring is poised to increase. For instance, a recent study developed an AI model to detect and track progression of Parkinson’s disease (for which there are no biomarkers) on the basis of nocturnal breathing signals using noninvasive, at-home assessment, providing evidence that AI may be useful in risk assessment before clinical diagnosis of the condition 29 , 30 . Additionally, digital atrial fibrillation screening by smart devices has been evaluated extensively in large-scale studies, including the Apple 31 , Huawei 32 and Fitbit 33 cardiac studies. Altogether, these siteless observational studies enrolled over 1 million participants, an amazing feat, and a randomized study showed the superiority of digital atrial fibrillation detection over usual care 34 .
Digital characterization and assessment of clinical status need to be standardized and harmonized, with interdisciplinary collaboration and regulatory input. Consensus is also needed to identify and characterize intermediate and surrogate endpoints for major chronic diseases. This requires specialty-specific incorporation of multiple levels of data such as genomic, proteomic and genotype–phenotype-based clinical data and disease-specific measurements, in addition to a layer of functional data 26 . The National Institutes of Health (NIH) and Food and Drug Administration (FDA) have developed BEST (Biomarkers, EndpointS and other Tools) resources to clarify the ambiguity in biomarkers and endpoints. This is a ‘living document’ that is continually updated as standards and evidence change 35 and that clarifies important definitions and describes some of the hierarchical relationships, connections and dependencies among the terms.
Clinical trial conduct
The components of clinical trial conduct are protocol implementation; patient selection, recruitment, monitoring and retention; ensuring compliance to safety reporting; and continuing review and data analysis. The pharmaceutical industry and the healthcare sector invest substantial resources into clinical trial conduct, but changes are urgently needed to make the process more seamless. Moreover, the pace at which clinical trials are conducted is too slow to match the research advances in every field; thus, a high-tech transformation of every component in a stepwise manner is needed.
One of the positive sides of the pandemic is that it forced the system to redirect clinical trials to be more patient-centric than before, thus giving more importance to the principal subject of clinical research—the patient 36 (Fig. 3 ). This has led to decentralized trials and digital, remote and ‘virtual’ trials (which allow patients access to trials regardless of their geographic location), as well as ‘hospital-at-home’ and home-based monitoring concepts 37 . Such rapid strides have been aided by guidance from regulatory authorities 38 . Adopting an AI-based approach to enhance the patient experience can further improve high-fidelity assessments and ensure compliance with protocols 39 . Although digitalization, virtualization and decentralization are not cures for clinical research crises, they can create efficiencies that may have a sizeable and long-term downstream impact.
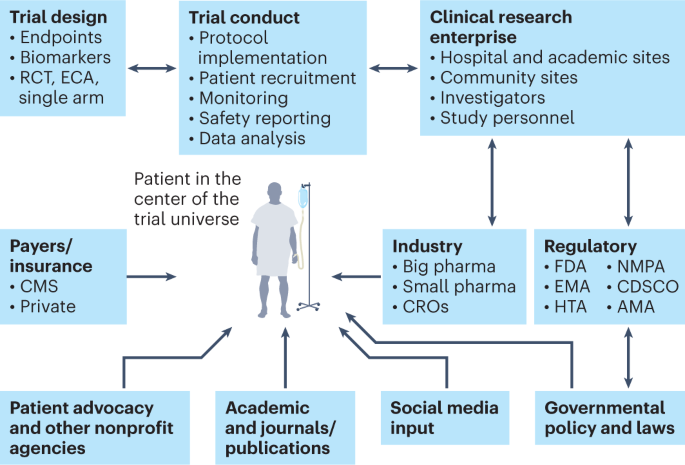
The main constituents of the clinical trial enterprise—patients, academic centers, industry sponsors (big and small pharma), government/cooperative group sponsors, regulatory agencies, patient advocacy organizations and CROs—need to work together, with the patient as the center of this clinical trial universe. AMA, African Medicines Agency; CDSCO, Central Drugs Standard Control Organization (India); CMS, Centers for Medicare and Medicaid Services; ECA, external control arm; EMA, European Medicines Agency; HTA, Health Technology Assessment; NMPA, National Medical Products Administration (China).
Physicians, healthcare team members and clinical investigators at academic sites and other trial enrolling sites contribute immensely to patient recruitment. In addition, high-impact, high-functioning sites (as in major academic centers of excellence) often have a portfolio of trials and screen patients presenting to the system in an efficient manner. Such sites are in the minority, however, and most clinical trial sites are challenged with staffing constraints and other barriers.
Clinical trial research enterprise
Efficiency and collaboration in the clinical trial research enterprise are major components of clinical trial success. The main constituents of the clinical trial enterprise are patients, academic centers, industry sponsors (big and small pharma), government/cooperative group sponsors, regulatory agencies, patient advocacy organizations and contract research organizations (CROs), and all of these need to work together with the patient as the center of the clinical trial universe (Fig. 3 ). Moreover, this whole system needs a digital overhaul as many sites still use protocol binders, pen-and-paper diaries, faxes between sites, unstructured data and decades-old software systems. Registrational clinical trials need to be well managed on a day-to-day basis with rigorous electronic data capture and monitoring. Integration of blockchain technology into the clinical trial management system could conceivably bolster trust in the clinical trial process and facilitate regulatory oversight 40 .
Patient participation in clinical trials is key, as there can be no trials without patients. Clinical trial organizers should make it easier for patients to participate in trials. In addition, physician–patient treatment decisions for major diseases should include clinical trial options as standard. These clinical trials should be easily accessible and should ensure that no patients are unnecessarily excluded; this can be achieved with site-agnostic clinical trial matching and navigation services. In addition, clinical trial training should be a part of medical education so that a diverse pool of trained investigators and personnel from the entire healthcare enterprise can be available for clinical research.
It is about time
Clinical development timelines for drug candidates are a race against time from when patents are filed to final FDA approval 41 . Drug development timelines, on average, are approximately 10 years (Fig. 1 ). The swiftness of the development of the COVID-19 mRNA vaccines and the oral COVID-19 treatment nirmatrelvir/ritonavir tablets, both of which were developed within a year using a ‘lightspeed approach’, should not be an outlier 42 . The lessons learned should provide a model for multiple therapeutic areas of unmet need. The two small molecules that hold the record for the shortest timeline in drug development, osimertinib for EGFR -mutant non-small-cell lung cancer (NSCLC) (984 days via accelerated approval) and elexacaftor for cystic fibrosis (1,043 days via the regular path) 41 , in nonpandemic times demonstrate that this is possible.
The regulatory logjams slowing drug development necessitated the creation of programs such as the FDA’s accelerated approval pathway, which was introduced in 1992 to address the HIV and AIDS crisis and has since benefitted highly specialized areas such as precision oncology 43 . Multiple programs have been created to shorten timelines for the premarket process, including priority review, fast-track designation, breakthrough designation and orphan designation 44 . Beyond these programs, however, the timelines are still slow and there is an urgent need to address this for all diseases as drug development speed is crucial for patients, physicians and drug development stakeholders alike.
Globalizing drug development, harmonization and transportability
Although the mandate of the FDA is to the US population, their influence is global and, functionally, the FDA is the de facto regulator for the world. Other regulatory authorities such as the European Medicines Agency, the National Medical Products Administration in China and the Central Drugs Standard Control Organization in India, which in total serve more than 3 billion of the world’s population, are also evolving as key players in the global pharmaceutical sector. In addition, the newly established African Medicines Agency was set up (in 2019) to speed up timelines for vaccines and medicine approvals and to improve access to drugs, especially for emerging infectious diseases endemic to the continent 45 . All of these agencies need to be able to stand alone. In addition, there is an urgent need for global harmonization across regulatory authorities to address the substantial inequities in access to medicines. Ideally, clinical trials for new therapies should be conducted globally, for access and generalizability 46 . However, the reality is that clinical trials, including RCTs, cannot be conducted in every country to generate specific evidence for that country’s population. Evidence generation using transportability analysis is gaining traction and refers to the ability to generalize inferences from a study sample in one country to a target population in another country where the study was not conducted 47 , 48 . Transportability analyses may offer some evidence of external validity with implications for local regulatory and health technology assessments 48 .
Evidence generation in clinical research
Clinical studies of rare diseases.
As scientific advances drive clinical trials forward, trials on cancers and many rare diseases are being designed and conducted in small genetically defined or biomarker-defined subsets. Moreover, new methods to generate evidence of clinical benefit may accelerate clinical trial conduct and provide individuals with rare diseases access to new therapeutic compounds. Rare diseases affect an estimated 263 million–446 million people globally at any given time and are increasingly becoming a huge public health burden 49 . Clinical trials in this context come with their own challenges stemming from the rarity of the conditions and incomplete natural history data 50 . However, remarkable advances in molecular biology coupled with legislation to spur orphan disease developmental therapeutics have led to progress. There is increasing regulatory flexibility to use programs such as the accelerated approval program, and there are case scenarios whereby trials have used external control arms based on RWD.
As an example, the FDA granted accelerated approval to alpelisib (Vijoice, Novartis) for adults and children over 2 years of age who require systemic therapy for PIK3CA-related overgrowth spectrum, which includes a group of rare disorders linked to mutations in the PIK3CA gene 51 . Interestingly, efficacy was evaluated using a retrospective chart review of RWD from EPIK-P1 ( NCT04285723 ), a single-arm clinical study in which individuals with PIK3CA-related overgrowth spectrum received alpelisib as part of an expanded access program for compassionate use. The application for this approval used the Real-Time Oncology Review pilot program 52 , which streamlined data submission before filing of the entire clinical application, and Assessment Aid 53 , a voluntary submission from the applicant to facilitate assessment by the FDA. As a result, this application was granted priority review, breakthrough designation and orphan drug designation 51 .
N-of-1 trials
In the era of individualized genomic medicine, N-of-1 trials are emerging as a tool to study potentially fatal rare diseases. The N-of-1 trial is a single-patient clinical trial using the individual person as a unit of investigation to evaluate the efficacy and/or adverse events of different interventions through objective data-driven criteria 54 . For example, an antisense oligonucleotide therapy was designed for, and evaluated in, a single patient who had a fatal genetic neurodegenerative disorder known as CLN7 neuronal ceroid lipofuscinosis (a form of Batten’s disease) 55 . Another patient (who happened to be a physician) with idiopathic Castleman’s disease refractory to IL-6-blocking therapy identified the causative molecular alteration in his own disease to develop a personalized therapy 56 . In yet another example, rapid dose escalation with a selective RET inhibitor was evaluated in a single patient with highly refractory medullary thyroid carcinoma, to overcome a resistance mechanism specific to that patient 57 .
These sensational new drug discovery–translation paradigms raise important questions, such as what level of evidence is needed before exposing a human to a new drug, what evidence this approach might generate for the next patient and what challenges might exist with generalizability 58 . The concept of medical analog patient-specific ‘digital twins’ is an emerging area of research that has the potential to combine polynomial data (mechanistic data, medical history, with the power of AI) and may perhaps serve to enhance N-of-1 trials in the future, to further personalize medicine 37 , 59 , 60 .
RWD and real-world evidence
One of the major criticisms of all clinical trial research is that clinical trials do not represent the ‘real-world’ population; often, the restrictive criteria of clinical trials and the limited analyses framed to answer specific questions may not apply to real-world patients. A wide gap therefore exists between the trial world and the real world, and attempts have been made to close this gap 61 . Conventional trials have been designed on the basis of the misconception that regulatory bodies may not accommodate more modern and diverse evidence from RWD, which is no longer the case 61 , 62 .
It is important to distinguish between RWD, which refers to data generated from routine, standard care of patients 62 , and real-world ‘evidence’ (RWE), which is the evidence generated from RWD regarding the potential use of a product. RWE is generated by trial designs or analysis and is not restricted to randomized trials; instead, it comes from pragmatic trials and prospective and/or retrospective observational studies 62 , 63 .
In this purview of RWD and RWE, all stakeholders look to regulators for guidance. Consequently, regulators have taken a hands-on approach and provided guidance and a comprehensive framework launched through the 21st Century Cures Act 62 , 64 . Moreover, the FDA uses RWD and RWE for postmarketing safety monitoring, and insurance agencies have started to use such data for coverage decisions 62 . This has been necessitated by rapidly accelerating data input from multiple streams and layers into electronic health records, as well as wearables and biosensors, in parallel with new analytical capabilities (multimodal AI) to analyze the vast amount of data.
Evidence from synthetic or external control arms
RCTs are considered the gold standard for drug development and evidence as they allow for estimation of treatment effects that can be assigned to the experimental arm of interest. The randomization in these studies curtails the concern for confounding bias by removing systematic imbalances between arms in measured and unmeasured prognostic factors 65 . However, advances in the genomics of rare diseases and the discovery of rare oncogene-driven cancers have led to specific targeted therapies, for which evaluation in RCTs may not be feasible or ethical and may delay patient access to promising or lifesaving therapies.
In such cases, synthetic control arms are emerging as options for generating comparator arms that can ‘mimic’ the comparator arms of RCTs. Synthetic control arms are external to the study in question, and most are derived from RWD 65 . Moreover, RWD are obtained from electronic health records, administrative claims data, natural history registries and patient-generated data from many sources, including wearable devices 65 . Synthetic control arms may also be generated from previous clinical trial data (single or pooled trials). This is an emerging area primed for innovation as so much data are now available from multiple sources.
NSCLC is increasingly being divided into small oncogene-driven subsets, making it more challenging to conduct randomized trials 66 , and recent developments in the NSCLC trial landscape illustrate the utility of synthetic control arms. For instance, RET fusions are genomic drivers in 1–2% of NSCLCs, and pralsetinib is a selective RET-targeted therapy showing promising responses even in individuals with advanced disease. The ARROW study ( NCT03037385 ) was a single-arm registrational trial, conducted globally, to evaluate pralsetinib in RET fusion-positive individuals with NSCLC 67 , 68 . This trial showed a relative survival benefit with the drug when compared to an external standard-of-care control arm consisting of RWD cohorts derived from two Flatiron Health databases 66 . A template for future studies of this nature using quantitative bias analyses showed that comparisons between the external control arm and the trial arm are robust and able to withstand issues such as data missingness, potentially poorer outcomes in RWD and residual confounding 66 . Overall, the study provided evidence in favor of pralsetinib as a first-line treatment for RET fusion-positive NSCLC.
The use of synthetic control arms can accelerate drug development, and initial skepticism about them arose mainly from a lack of precedence and direction from regulatory authorities. These concerns are now being dispelled as synthetic control arms have been used recently for drug approvals for ultra-rare diseases. For example, neurofibromatosis is a rare disease seen in 1 in 3,000 births. Patients develop plexiform neurofibroma lesions that are painful and debilitating, causing motor and neuronal dysfunction. The MEK inhibitor selumetinib was approved for pediatric patients with symptomatic, inoperable plexiform neurofibromas on the basis of a dataset of 50 patients from Selumetinib in Pediatric Neurofibroma Trial (SPRINT)—a single-arm phase 2 trial showing a durable objective response rate and improvements in functional symptoms 65 , 69 , 70 . Comparator arms from two previously conducted trials provided evidence for the natural history of the disease and were submitted as an external control arm, which helped confirm that spontaneous regressions were uncommon and that the observed responses and symptom improvement represented a genuine treatment effect 69 .
Despite this progress, external control arms are still an emerging concept and they have mainly been used to investigate the natural history of disease and have not generally been included as primary evidence or in product labels. However, in the future, I can envision such comparative effectiveness analysis and comparator arms as primary evidence to support drug approval. Challenges mainly arise from data quality and data missingness, as well as uncertainty of whether external control data are fit for purpose. However, some of these concerns can be mitigated by quantitative bias analysis and other methodologies 66 , 71 .
Pediatric clinical trials
Although pediatric research has been at the forefront of major advances in medicine (extracorporeal membrane oxygenation 72 is a notable example) and has pushed the boundaries of modern oncology (for instance, in treating pediatric leukemia), innovations in new drug development are often delayed. Many rare and orphan diseases occur mainly in the pediatric population, and drug development in this population has always been operationally, ethically, statistically and methodologically challenging 73 , 74 . This is compounded by the limited understanding of basic biology, the ontology of disease manifestations, and the acute and long-term safety of products 73 , 74 . In addition, there is considerable off-label use of products in very young children, infants and neonates where clinical trials have not been feasible, and it is imperative that high-level evidence be generated by creative methods. Programs such as the Best Pharmaceuticals for Children Act (in 2002) and the Pediatric Research Equity Act (in 2003), made permanent in 2012 under the FDA Safety and Innovation Act, have incentivized and enhanced the development of pediatric therapeutics 73 . Innovative trial designs, RWD and leveraging data from other resources may help with risk–benefit assessment and drug approval, such as the approval for neurofibromatosis type 1 (NF1) 73 .
Reimagining the future of clinical trials
The landscape of AI in medicine has transformed recently, and AI is poised to become ubiquitous. Several RCTs have quantified the benefits of AI in specialties that use pattern recognition and interpretation of images, such as radiology (mammography and lung cancer screening), cardiology (interpreting electrocardiograms (EKGs), cardiac functional assessment and atrial fibrillation screening), gastroenterology (interpreting colonoscopies), pathology (cancer diagnosis), neurology (tracking disease evolution of amyotrophic lateral sclerosis and Parkinson’s disease), dermatology (diagnosing lesions) and ophthalmology (eye disease screening) 75 . However, most AI research focuses on ‘clinical care delivery’ applications and not ‘clinical trial research’ 76 .
The integration of AI into clinical trial research has been slower than expected, mainly owing to the (perceived) friction between AI versus human intelligence. Nevertheless, trials of data generation and interpretation should be conducted, and AI should be used to augment human intelligence—not seen as something to replace it 77 . Next-generation clinical trials using AI should consider AI + human rather than AI versus human scenarios 75 , 78 . The clinical trial guidelines for protocols (Standard Protocol Items: Recommendations for Interventional Trials–Artificial Intelligence (SPIRIT-AI) extension) and publications (Consolidated Standards of Reporting Trials–Artificial Intelligence (CONSORT-AI) extension) 79 , 80 are intended to achieve standardized and transparent reporting for randomized clinical trials involving AI, and these are just the beginning of a new phase of clinical research modernization.
Given the time and cost involved in developing a drug, every failed drug in the market represents a considerable loss to the drug development ecosystem. In addition, inferior trial designs, suboptimal patient recruitment, poor infrastructure to run trials, and inefficiency in trial conduct and monitoring have plagued the system for decades. AI has the potential to augment all phases of drug development, from drug design to the complete drug development cycle (Fig. 1 ).
Clinical trial conduct is still rudimentary in many ways. For instance, in oncology trials, a few aspects of two-dimensional lesions are measured and followed over time and effectiveness of the drug is evaluated by shrinkage of these lesions. Automated quantitative assessments and artificial neural networks can aid in automated rapid processing of multiple lesions 81 . In cardiology trials, vital signs are measured once a week in clinic, and, in neurology, patient questionnaires are administered in clinic. Now, these data can all be tracked dynamically in real time using wearable sensor technology. The application of AI to such areas can have a transformational near-term impact. In addition, pattern recognition using deep neural networks can help with reading scans, pathology images and EKGs, among others 37 , 78 .
The current evidence-based medicine pyramid represents the tip of the iceberg and barely provides shallow evidence to care for a generic patient (Fig. 4 ). Hence, a deep synthesis and amalgamation of all available data is needed to achieve next-generation, ‘deep’ evidence-based medicine. The main challenge in the next two decades will be to tap the potential of multidimensional evidence generation 82 by extracting, collating and mining large sets of natural history data, genomics and all other omics analysis, all published clinical studies, RWD, data from ubiquitous smart devices and amassed data from the IoMT to provide next-generation evidence for deep medicine.
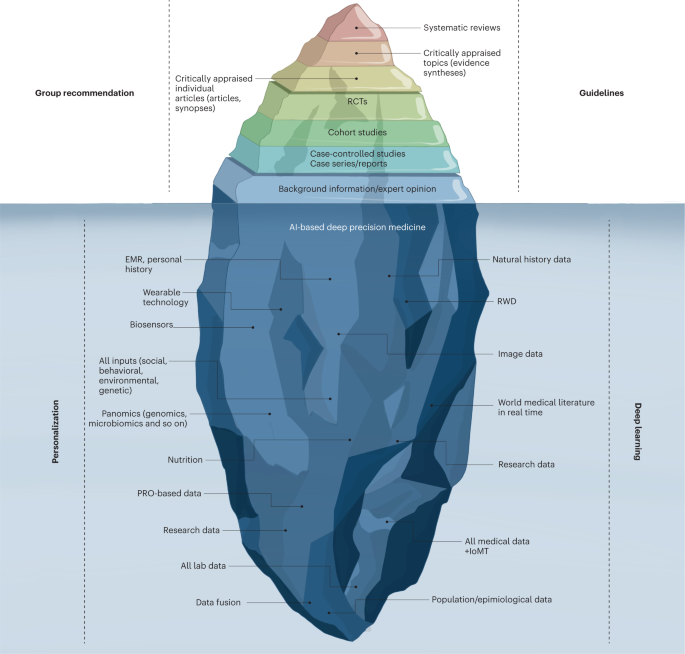
The current evidence-based medicine (EBM) pyramid represents the tip of the iceberg and barely provides enough shallow evidence to care for a generic patient. Hence, a deep synthesis and amalgamation of all available data is needed to achieve next-generation, deep evidence-based medicine. The main challenge ahead in the next two decades will be extracting, collating and mining large sets of natural history data, genomics and all omics analyses, all published clinical studies, RWD and amassed data from the IoMT to provide next-generation evidence for deep medicine. PRO, patient-reported outcomes.
Partnerships in drug development
Currently, the pharma industry is the main driver of drug development, and their expenditures far exceed investments from any national agency such as the National Institutes of Health 61 . There are two domains of clinical trials. The first of these is from ‘big pharma’, which uses CROs to run trials; such trials are very often approved for registration by the FDA. The second domain encompasses academic clinical trials, which often operate on a very limited budget, do not often evaluate new compounds and, thus, rarely result in FDA registration. In this era of reduced federal funding for research, more partnerships are needed for drug development. Academic centers and community sites are crucial for patient enrollment; however, a siloed mentality has impacted drug development and delayed access to lifesaving therapies. Therefore, collaborations among specific disease organizations, academic institutions, federal agencies and patient advocacy groups are crucial for betterment of the health of populations (Fig. 3 ). Because the pharma industry is hesitant to invest huge amounts with limited financial return, especially in rare diseases, federal agencies have developed programs to incentivize rare disease drug development 1 . Moreover, disease-focused organizations have collaborated with the pharma industry, federal agencies and academia to form ‘venture philanthropy’ with risk-sharing financial models to de-risk drug development 1 . Many academic institutions are entering into risk-sharing strategic alliances with the pharma industry to collaborate across preclinical and clinical development phases. Such successful innovative partnership models have set a precedent in diseases such as cystic fibrosis, multiple myeloma, type 1 diabetes mellitus and other rare diseases 1 . These collaborations have effectively catalyzed innovation through all phases of drug development and provided a compelling reason to sustain and foster more of these sorts of programs.
Social media and online community research
Social media outlets (Twitter, Facebook and so on) can influence patient accrual in clinical trials. They can strongly influence and address historical clinical trial challenges, including the lack of awareness among patients and physicians about available trials and the lack of community engagement. More than 4.48 billion people use social media globally, and this number is projected to increase to almost 6 billion in 2027 (ref. 83 ). Over 70% of Americans are on social media, including rural dwellers and adolescent and young adult populations who have always been under-represented in clinical trials. Although many older adults do not use social media, their caregivers are likely to.
People with terminal diseases often self-experiment with drugs, and online patient communities can provide environments for sharing and monitoring such drug usage. This can allow for observational studies to be planned around quantitative, internet-based outcome data. For example, researchers developed an algorithm to dissect the data reported on the PatientsLikeMe website by people with amyotrophic lateral sclerosis who experimented with lithium carbonate treatment 84 . This analysis reached the same conclusion as an ensuing RCT, suggesting that data from online patient behavior can help accelerate drug development and evaluate the effectiveness of drugs already in use.
An increase in engagement from patients and patient advocacy groups can aid patient education and outreach and can facilitate patient-partnered research, as well as allowing for incorporation of patients’ perspectives in the design of clinical research—ultimately generating research that is driven by the needs of real people with the disease under investigation. Moreover, social media breaks open silos dividing researchers and clinicians, creating enormous potential to influence all areas of medicine 85 .
The success of future clinical trials requires a fundamental transformation in how trials are designed, conducted, monitored, adapted, reported and regulated to generate the best evidence. The status quo model is unsustainable. Instead, preventive, personalized, pragmatic and patient-participatory medicine is needed, and paradigm shifts are required to get there via sustainable growth. Silos need to be broken. Standards of care and clinical trials are currently viewed in different realms; however, the overarching goal of both is to improve health outcomes. The COVID-19 pandemic created an opportunity to observe how routine clinical care and clinical trials can work synergistically to generate evidence 86 . Pragmatic platform trials such as the RECOVERY trial should be a model and guide for trial efficiency and real-time impact.
Current paradigms must be continuously challenged by emerging technology and by all stakeholders (the new generations of scientists, physicians, the pharma industry, regulatory authorities and, most importantly, patients). Disruptive innovation should lead to every clinical site being a research site, with all necessary quality checks and research as part of the standard of care. The healthcare system should be integrated into an intuitive RWE-generation system, with clinical research and clinical care going hand in hand. Beyond an ad hoc creative flash of genius (necessitated by a pandemic), sustained momentum will be needed to leverage the knowledge gained from programs such as ‘Operation Warp Speed’ (initiated by the US government to accelerate COVID-19 vaccine development). My personal view is that every major disease needs a ‘Moonshot’ program and every rare disease should have an ‘Operation Warp Speed’—both with clearly identified, sustainable goals to improve population health and address equity, diversity and global access to therapies. Methodological advances and future AI-based analyses of all data will provide deep evidence to realize the goal of personalized medicine— that is, to offer the right treatment to the right patient at the right time.
Ramsey, B. W., Nepom, G. T. & Lonial, S. Academic, foundation, and industry collaboration in finding new therapies. N. Engl. J. Med. 376 , 1762–1769 (2017).
Article CAS Google Scholar
Butler, D. Translational research: crossing the valley of death. Nature 453 , 840–842 (2008).
DiMasi, J. A., Grabowski, H. G. & Hansen, R. W. Innovation in the pharmaceutical industry: new estimates of R&D costs. J. Health Econ. 47 , 20–33 (2016).
Article Google Scholar
Wouters, O. J., McKee, M. & Luyten, J. Estimated research and development investment needed to bring a new medicine to market, 2009–2018. JAMA 323 , 844–853 (2020).
Subbiah, V. A global effort to understand the riddles of COVID-19 and cancer. Nat. Cancer 1 , 943–945 (2020).
Flaherty, K. T. et al. Rethinking cancer clinical trial conduct induced by COVID-19: an academic center, industry, government, and regulatory agency perspective. Cancer Discov. 11 , 1881–1885 (2021).
Samimi, G. et al. Lessons learned from the impact of COVID-19 on NCI-sponsored cancer prevention clinical trials: moving toward participant-centric study designs. Cancer Prev. Res. 15 , 279–284 (2022).
National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP). Health and Economic Costs of Chronic Diseases https://www.cdc.gov/chronicdisease/programs-impact/pop/index.htm (2022).
Menta, A. K., Subbiah, I. M. & Subbiah, V. Bringing wearable devices into oncology practice: fitting smart technology in the clinic. Discov. Med. 26 , 261–270 (2018).
Google Scholar
Krittanawong, C., Johnson, K. W. & Tang, W. W. How artificial intelligence could redefine clinical trials in cardiovascular medicine: lessons learned from oncology. Per. Med. 16 , 83–88 (2019).
Subbiah, V. & Kurzrock, R. Challenging standard-of-care paradigms in the precision oncology era. Trends Cancer 4 , 101–109 (2018).
Woodcock, J. & LaVange, L. M. Master protocols to study multiple therapies, multiple diseases, or both. N. Engl. J. Med. 377 , 62–70 (2017).
Park, J. J. H. et al. Systematic review of basket trials, umbrella trials, and platform trials: a landscape analysis of master protocols. Trials 20 , 572 (2019).
Dickson, D. et al. The master observational trial: a new class of master protocol to advance precision medicine. Cell 180 , 9–14 (2020).
Das, S. & Lo, A. W. Re-inventing drug development: a case study of the I-SPY 2 breast cancer clinical trials program. Contemp. Clin. Trials 62 , 168–174 (2017).
Redman, M. W. et al. Biomarker-driven therapies for previously treated squamous non-small-cell lung cancer (Lung-MAP SWOG S1400): a biomarker-driven master protocol. Lancet Oncol. 21 , 1589–1601 (2020).
Subbiah, V. et al. Pan-cancer efficacy of vemurafenib in BRAF V600 -mutant non-melanoma cancers. Cancer Discov. 10 , 657–663 (2020).
Subbiah, V. et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600 -mutant anaplastic thyroid cancer. J. Clin. Oncol. 36 , 7–13 (2018).
Subbiah, V. et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion-positive solid tumors from the phase 1/2 ARROW trial. Nat. Med. 28 , 1640–1645 (2022).
Drilon, A. et al. Efficacy of selpercatinib in RET fusion–positive non–small-cell lung cancer. N. Engl. J. Med. 383 , 813–824 (2020).
Subbiah, V. et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial. Lancet Oncol. 23 , 1261–1273 (2022).
Normand, S.-L. T. The RECOVERY platform. N. Engl. J. Med. 384 , 757–758 (2020).
Turner, N. C. et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): a multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 21 , 1296–1308 (2020).
Gold, S. M. et al. Platform trials and the future of evaluating therapeutic behavioural interventions. Nat. Rev. Psychol. 1 , 7–8 (2022).
Dickson, D. et al. Snapshot: trial types in precision medicine. Cell 181 , 208 (2020).
Ochoa, D. et al. Human genetics evidence supports two-thirds of the 2021 FDA-approved drugs. Nat. Rev. Drug Discov. 21 , 551 (2022).
Wickström, K. & Moseley, J. Biomarkers and surrogate endpoints in drug development: a european regulatory view. Invest. Ophthalmol. Vis. Sci. 58 , BIO27–BIO33 (2017).
Robb, M. A., McInnes, P. M. & Califf, R. M. Biomarkers and surrogate endpoints: developing common terminology and definitions. JAMA 315 , 1107–1108 (2016).
Landers, M., Dorsey, R. & Saria, S. Digital endpoints: definition, benefits, and current barriers in accelerating development and adoption. Digit. Biomark. 5 , 216–223 (2021).
Yang, Y. et al. Artificial intelligence-enabled detection and assessment of Parkinson’s disease using nocturnal breathing signals. Nat. Med. 28 , 2207–2215 (2022).
Perez, M. V. et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N. Engl. J. Med. 381 , 1909–1917 (2019).
Guo, Y. et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J. Am. Coll. Cardiol. 74 , 2365–2375 (2019).
Lubitz, S. A. et al. Rationale and design of a large population study to validate software for the assessment of atrial fibrillation from data acquired by a consumer tracker or smartwatch: the Fitbit Heart Study. Am. Heart J. 238 , 16–26 (2021).
Rizas, K. D. et al. Smartphone-based screening for atrial fibrillation: a pragmatic randomized clinical trial. Nat. Med. 28 , 1823–1830 (2022).
FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource https://www.ncbi.nlm.nih.gov/books/NBK326791/ (2016).
Desai, A. & Subbiah, V. COVID-19 pandemic and cancer clinical trial pandemonium: finding the silver lining. J. Immunother. Precis. Oncol. 4 , 64–66 (2020).
Acosta, J. N., Falcone, G. J., Rajpurkar, P. & Topol, E. J. Multimodal biomedical AI. Nat. Med. 28 , 1773–1784 (2022).
Food and Drug Administration. Digital health technologies for remote data acquisition in clinical investigations, draft guidance for industry, investigators, and other stakeholders. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/digital-health-technologies-remote-data-acquisition-clinical-investigations (2022).
Thomas, K. A. & Kidziński, Ł. Artificial intelligence can improve patients’ experience in decentralized clinical trials. Nat. Med. https://doi.org/10.1038/s41591-022-02034-4 (2022).
Wong, D. R., Bhattacharya, S. & Butte, A. J. Prototype of running clinical trials in an untrustworthy environment using blockchain. Nat. Commun. 10 , 917 (2019).
Brown, D. G., Wobst, H. J., Kapoor, A., Kenna, L. A. & Southall, N. Clinical development times for innovative drugs. Nat. Rev. Drug Discov. 21 , 793–794 (2021).
Anderson, A. S. A lightspeed approach to pandemic drug development. Nat. Med. 28 , 1538 (2022).
Subbiah, V. et al. Accelerated approvals hit the target in precision oncology. Nat. Med. 28 , 1976–1979 (2022).
Kepplinger, E. E. FDA’s expedited approval mechanisms for new drug products. Biotechnol. Law Rep. 34 , 15–37 (2015).
Ncube, B. M., Dube, A. & Ward, K. Establishment of the African Medicines Agency: progress, challenges and regulatory readiness. J. Pharm. Policy Pract. 14 , 29 (2021).
Moyers, J. T. & Subbiah, V. Think globally, act locally: globalizing precision oncology. Cancer Discov. 12 , 886–888 (2022).
Degtiar, I. & Rose, S. A review of generalizability and transportability. Annu. Rev. Stat. Appl. 10 , 1 (2023).
Ramagopalan, S. V. et al. Transportability of overall survival estimates from US to Canadian patients with advanced non–small cell lung cancer with implications for regulatory and health technology assessment. JAMA Netw. Open 5 , e2239874 (2022).
Nguengang Wakap, S. et al. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur. J. Hum. Genet. 28 , 165–173 (2020).
Pizzamiglio, C., Vernon, H. J., Hanna, M. G. & Pitceathly, R. D. S. Designing clinical trials for rare diseases: unique challenges and opportunities. Nat. Rev. Methods Primers 2 , 13 (2022).
Food and Drug Administration. FDA approves alpelisib for PIK3CA-related overgrowth spectrum. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-alpelisib-pik3ca-related-overgrowth-spectrum (2022).
Food and Drug Administration. Real-time oncology review. https://www.fda.gov/about-fda/oncology-center-excellence/real-time-oncology-review (2022).
Food and Drug Administration. Assessment aid. https://www.fda.gov/about-fda/oncology-center-excellence/assessment-aid (2022).
Lillie, E. O. et al. The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Per. Med. 8 , 161–173 (2011).
Kim, J. et al. Patient-customized oligonucleotide therapy for a rare genetic disease. N. Engl. J. Med. 381 , 1644–1652 (2019).
Fajgenbaum, D. C. et al. Identifying and targeting pathogenic PI3K/AKT/mTOR signaling in IL-6 blockade–refractory idiopathic multicentric Castleman disease. J. Clin. Investig. 129 , 4451–4463 (2019).
Subbiah, V. et al. Selective RET kinase inhibition for patients with RET -altered cancers. Ann. Oncol. 29 , 1869–1876 (2018).
Woodcock, J. & Marks, P. Drug regulation in the era of individualized therapies. N. Engl. J. Med. 381 , 1678–1680 (2019).
Björnsson, B. et al. Digital twins to personalize medicine. Genome Med. 12 , 4 (2019).
Laubenbacher, R., Sluka, J. P. & Glazier, J. A. Using digital twins in viral infection. Science 371 , 1105–1106 (2021).
Sherman, R. E., Davies, K. M., Robb, M. A., Hunter, N. L. & Califf, R. M. Accelerating development of scientific evidence for medical products within the existing US regulatory framework. Nat. Rev. Drug Discov. 16 , 297–298 (2017).
Concato, J. & Corrigan-Curay, J. Real-world evidence—where are we now? N. Engl. J. Med. 386 , 1680–1682 (2022).
Concato, J., Stein, P., Dal Pan, G. J., Ball, R. & Corrigan-Curay, J. Randomized, observational, interventional, and real-world—what’s in a name? Pharmacoepidemiol. Drug Saf. 29 , 1514–1517 (2020).
Food and Drug Administration. Real-world evidence. https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence (2022).
Mishra-Kalyani, P. S. et al. External control arms in oncology: current use and future directions. Ann. Oncol. 33 , 376–383 (2022).
Popat, S. et al. Addressing challenges with real-world synthetic control arms to demonstrate the comparative effectiveness of pralsetinib in non-small cell lung cancer. Nat. Commun. 13 , 3500 (2022).
Gainor, J. F. et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 22 , 959–969 (2021).
Subbiah, V. et al. Pralsetinib for patients with advanced or metastatic RET -altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 9 , 491–501 (2021).
Center for Drug Evaluation and Research, Food and Drug Administration. NDA multi-disciplinary review and evaluation: NDA 213756 Koselugo (selumetinib). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213756Orig1s000MultidisciplineR.pdf (2020).
Casey, D. et al. FDA approval summary: selumetinib for plexiform neurofibroma. Clin. Cancer Res. 27 , 4142–4146 (2021).
Wilkinson, S. et al. Assessment of alectinib vs ceritinib in ALK-positive non–small cell lung cancer in phase 2 trials and in real-world data. JAMA Netw. Open 4 , e2126306 (2021).
Bartlett, R. H. et al. Extracorporeal membrane oxygenation (ECMO) in neonatal respiratory failure. 100 cases. Ann. Surg. 204 , 236–245 (1986).
McCune, S. & Portman, R. J. Innovation and opportunities in pediatric therapeutic development. Ther. Innov. Regul. Sci. 53 , 564–566 (2019).
Subbiah, V. Fast-tracking novel drugs in pediatric oncology. Cell Cycle 14 , 1127–1128 (2015).
Rajpurkar, P., Chen, E., Banerjee, O. & Topol, E. J. AI in health and medicine. Nat. Med. 28 , 31–38 (2022).
Weissler, E. H. et al. The role of machine learning in clinical research: transforming the future of evidence generation. Trials 22 , 537 (2021).
Adashek, J. J., Subbiah, I. M. & Subbiah, V. Artificial intelligence systems assisting oncologists? Resist and desist or enlist and coexist. Oncologist 24 , 1291–1293 (2019).
Topol, E. J. High-performance medicine: the convergence of human and artificial intelligence. Nat. Med. 25 , 44–56 (2019).
Liu, X. et al. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Nat. Med. 26 , 1364–1374 (2020).
Cruz Rivera, S. et al. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. Nat. Med. 26 , 1351–1363 (2020).
Kickingereder, P. et al. Automated quantitative tumour response assessment of MRI in neuro-oncology with artificial neural networks: a multicentre, retrospective study. Lancet Oncol. 20 , 728–740 (2019).
Jarow, J. P., LaVange, L. & Woodcock, J. Multidimensional evidence generation and FDA regulatory decision making: defining and using “real-world” data. JAMA 318 , 703–704 (2017).
Dean, B. Social network usage & growth statistics: how many people use social media in 2022? Backlinko https://backlinko.com/social-media-users (2021).
Wicks, P., Vaughan, T. E., Massagli, M. P. & Heywood, J. Accelerated clinical discovery using self-reported patient data collected online and a patient-matching algorithm. Nat. Biotechnol. 29 , 411–414 (2011).
Morgan, G. et al. The (r)evolution of social media in oncology: engage, enlighten, and encourage. Cancer Discov. 12 , 1620–1624 (2022).
Pessoa-Amorim, G. et al. Making trials part of good clinical care: lessons from the RECOVERY trial. Future Healthc. J. 8 , e243–e250 (2021).
Download references
Acknowledgements
V.S. is an Andrew Sabin Family Foundation fellow at the University of Texas MD Anderson Cancer Center. V.S. acknowledges the support of the Jacquelyn A. Brady Fund. V.S. thanks the team at Draw Impacts for figures. V.S. is supported by the US National Institutes of Health (NIH) (grants R01CA242845 and R01CA273168); the MD Anderson Cancer Center Department of Investigational Cancer Therapeutics is supported by the Cancer Prevention and Research Institute of Texas (grant RP1100584), the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy (grant 1U01CA180964), NCATS (Center for Clinical and Translational Sciences) (grant UL1TR000371) and the MD Anderson Cancer Center Support (grant P30CA016672).
Author information
Authors and affiliations.
Department of Investigational Cancer Therapeutics, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Vivek Subbiah
Division of Pediatrics, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
MD Anderson Cancer Network, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Vivek Subbiah .
Ethics declarations
Competing interests.
None relevant to the manuscript. V.S. reports research funding/grant support for clinical trials from AbbVie, Agensys, Alfasigma, Altum, Amgen, Bayer, BERG Health, Blueprint Medicines, Boston Biomedical, Boston Pharmaceuticals, Celgene, D3 Bio, Dragonfly Therapeutics, Exelixis, Fujifilm, GlaxoSmithKline, Idera Pharmaceuticals, Incyte, Inhibrx, Loxo Oncology, MedImmune, MultiVir, NanoCarrier, National Comprehensive Cancer Network, NCI-CTEP, Northwest Biotherapeutics, Novartis, PharmaMar, Pfizer, Relay Therapeutics, Roche/Genentech, Takeda, Turning Point Therapeutics, UT MD Anderson Cancer Center and Vegenics; travel support from ASCO, ESMO, Helsinn Healthcare, Incyte, Novartis and PharmaMar; consultancy/advisory board participation for Helsinn Healthcare, Jazz Pharmaceuticals, Incyte, Loxo Oncology/Eli Lilly, MedImmune, Novartis, QED Therapeutics, Relay Therapeutics, Daiichi-Sankyo and R-Pharm US; and a relationship with Medscape.
Peer review
Peer review information.
Nature Medicine thanks Benedikt Westphalen, Edward Mills and Christina Yap for their contribution to the peer review of this work. Primary Handling Editor: Karen O’Leary, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Subbiah, V. The next generation of evidence-based medicine. Nat Med 29 , 49–58 (2023). https://doi.org/10.1038/s41591-022-02160-z
Download citation
Received : 28 September 2022
Accepted : 11 November 2022
Published : 16 January 2023
Issue Date : January 2023
DOI : https://doi.org/10.1038/s41591-022-02160-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Mapping the landscape of research on insulin resistance: a visualization analysis of randomized clinical trials.
- Sa’ed H. Zyoud
Journal of Health, Population and Nutrition (2024)
Nurse managers’ managerial innovation and it’s relation to proactivity behavior and locus of control among intensive care nurses
- Loly Mohamed Shawky Elbus
- Mohamed Gamal Mostafa
- Seham Aly Mahmoud
BMC Nursing (2024)
Overcoming the barriers to better evidence generation from clinical trials
- Lindsay Kehoe
- Trevan Locke
Trials (2024)
Pharmacological reactivation of p53 in the era of precision anticancer medicine
- Charlotte Strandgren
- Klas G. Wiman
Nature Reviews Clinical Oncology (2024)
A guide to artificial intelligence for cancer researchers
- Raquel Perez-Lopez
- Narmin Ghaffari Laleh
- Jakob Nikolas Kather
Nature Reviews Cancer (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

Research on Biomedical Engineering
Research on Biomedical Engineering is an online, peer-reviewed journal dedicated to all fields of Biomedical Engineering.
- Multidisciplinary in nature, catering to readers and authors interested in developing tools based on engineering and physical sciences to solve biological and medical problems.
- Publishes Original Research Articles, Reviews, and Technical Communications.
- Covers a wide range of contributing areas including, metrology and biomedical engineering, health technology, health informatics and telemedicine, biotechnology, artificial organs, implants and biomaterials, proteomics, genomics and bioinformatics, biomedical instrumentation, biomechanics, rehabilitation engineering and assistive technologies, biomedical signal processing, modelling of physiological systems, use of laser in health, use of ultrasound in health, use of radiation in health and medical imaging.
- The official journal of the Brazilian Society of Biomedical Engineering.
- Alcimar Barbosa Soares
Societies and partnerships
Latest issue
Volume 40, Issue 2
Latest articles
Unveiling the potential of confocal raman spectroscopy in the analysis of oil permeation in human hair fibers.
- Liliane Trivellato Grassi
- Vera Mileide Trivellato Grassi
- Airton A. Martin

Calcium aluminate cement blended to bioactive glass and strontium: in vitro and in vivo evaluation studies
- Isabela dos Santos Gonçalves
- Giovanni Moreira Donda
- Ivone Regina de Oliveira

A robust transfer learning approach for colorectal cancer identification based on histopathology images
- Toto Haryanto
- Helmi Al Farel
- Ari Wibisono

Extracorporeal membrane oxygenation versus conventional therapy in patients with acute respiratory failure: systematic review with meta-analysis
- Daniel Baldoino de Souza
- Cassiana Gabriela Lima Barreto
- Adriano Alves Pereira

Analysis of fractal dimension of lymphocytes exposed to different doses of ionizing radiation through box counting method
- Jonas Sérgio de Oliveira Filho
- Anna Beatriz de Oliveira Barbosa
- Isvânia Maria Serafim da Silva Lopes

Journal updates
Special issue: 'emerging technologies for fighting covid-19, special issue form and guest editor guidelines.
A Special Issue Form and specific information for Guest Editors can be found here.
Top 10 Downloaded Articles 2023
Follow us on facebook, x, and linkedin.
Springer Engineering is on Facebook, X, and LinkedIn. Follow us and keep up to date with the latest journal news, research highlights, and more.
Journal information
- EI Compendex
- Google Scholar
- Japanese Science and Technology Agency (JST)
- OCLC WorldCat Discovery Service
- TD Net Discovery Service
- UGC-CARE List (India)
Rights and permissions
Editorial policies
© Sociedade Brasileira de Engenharia Biomedica
- Find a journal
- Publish with us
- Track your research
IEEE Account
- Change Username/Password
- Update Address
Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.

COMMENTS
Journal of Biomedical Science publishes molecular studies on basic medical sciences. Find the latest articles on cancer, epigenetics, and more.
by Zeiger, Mimi Publication date 1991 Topics Medical writing -- Handbooks, manuals, etc, Technical writing -- Handbooks, manuals, etc, Writing Publisher New York : McGraw-Hill, Health Professions Division Collection internetarchivebooks; inlibrary; printdisabled Contributor Internet Archive Language English Item Size 992.5M x, 422 pages : 28 cm Includes bibliographical references (p. 411-413 ...
PMC is a free archive of biomedical and life sciences literature from PubMed. Search, browse and access full-text articles from journals and books.
View PDF. 33 Essentials of Writing Biomedical Research Papers. Mimi Zeiger. New York: McGraw-Hill, Health Professions Div., 1991. Reviewed by Lilita Rodman University of British Columbia This is an in-depth study of a very specific genre, the biomedical research paper. Growing out of courses Zeiger has taught to graduate students, postdoctoral ...
Read this chapter of Essentials of Writing Biomedical Research Papers, 2e online now, exclusively on AccessBiomedical Science. AccessBiomedical Science is a subscription-based resource from McGraw Hill that features trusted medical content from the best minds in medicine.
Essentials of Writing Biomedical Research Papers is extremely detailed and analytical, and it contains many useful exercises. I recommend delving into particular sections and concentrating on specific areas—for example, writing abstracts, choosing a title, presenting data—rather than to work through the book from start to end.
Overview. Focuses very precisely on the biomedical research article. Biomedical language is quite different in many respects from general English and other scientific dialects, and so biomedical language deserves to be treated separately. Derived largely from analyses of biomedical corpora. Hence, the advice offered is evidence-based rather ...
Read Essentials of Writing Biomedical Research Papers, 2e online now, exclusively on AccessBiomedical Science. AccessBiomedical Science is a subscription-based resource from McGraw Hill that features trusted medical content from the best minds in medicine.
Provides immediate help for anyone preparing a biomedical paper by givin specific advice on organizing the components of the paper, effective writing techniques, writing an effective results sections, documentation issues, sentence structure and much more. The new edition includes new examples from the current literature including many involving molecular biology, expanded exercises at the end ...
Essentials of Writing Biomedical Research Papers. M. Zeiger. Published 25 July 1991. Medicine, Biology. TLDR. This paper aims to provide a scaffolding for clear writing in the field of biomedical research paper-writing by demonstrating the importance of sentence structure in the development of narrative structure.
Read chapter 5 of Essentials of Writing Biomedical Research Papers, 2e online now, exclusively on AccessBiomedical Science. AccessBiomedical Science is a subscription-based resource from McGraw Hill that features trusted medical content from the best minds in medicine.
Writing Biomedical Research Papers for Publication November 2019 DOI: 10.13140/RG.2.2.19649.92006 Conference: American Medical Writers' Medical Writing and Communication Conference Authors ...
As the author says in the preface, the aim of the book is "to explain and illustrate what a clearly written biomedical paper is." Although methods papers -- "papers that report new or improved methods, apparatus, or materials" -- receive some C1ttention, the focus is "the journal article that reports results of original research." Theoretical papers, case reports, and review articles are ...
Journal publication is an integral part of research that documents new information gleaned from an investigation, and in so doing inspires more research, in an endless search for truth. The amount of new biomedical literature that the world gets bombarded with these days may sometimes seem overwhelming. Yet, research results need to be reported in peer-reviewed journals and researchers need to ...
Biomedical engineering advances: A review of innovations in healthcare and patient outcomes January 2024 International Journal of Science and Research Archive 11 (1):870-882 11 (1):870-882 DOI: 10 ...
Over the next decade, the application of machine learning, deep neural networks and multimodal biomedical AI is poised to reinvigorate clinical research from all angles, including drug discovery ...
Auckland, New Zealand. Specially designed for aspiring researchers, this book presents a systematic. exposition of the basic principles and methodologies involved in biomedical research. The book ...
Research on Biomedical Engineering is an online, peer-reviewed journal dedicated to all fields of Biomedical Engineering. Multidisciplinary in nature, catering to readers and authors interested in developing tools based on engineering and physical sciences to solve biological and medical problems. Publishes Original Research Articles, Reviews ...
This paper is, in effect, an essay looking toward the improvement of instruments used in biomedical research, their availability, and the knowledge and skills necessary to use them to the best advantage. The discussion suggests that it might be desirable to establish a center or centers where teaching, research, and engineering can be done on instruments; where scientists or technicians can ...
1 Basic Course in Biomedical Research Handbook Dr. T anmay Mehta MD-DNB-MNAMS Microbiology, Advanced Course in Medical Education (ACME), Post Graduate Diploma in Clinical Trial Management (PGDCTM ...