Foundations of Clinical Research
This Harvard Medical School six-month, application-based certificate program provides the essential skill sets and fundamental knowledge required to begin or expand your clinical research career.


Associated Schools

Harvard Medical School
What you'll learn.
Understand and apply the foundational concepts of biostatistics and epidemiology
Develop a research question and formulate a testable hypothesis
Design and begin to implement a clinical research study
Cultivate the skills required to present a clinical research study
Critically evaluate the research findings in medical literature
Synthesize crucial statistical analyses using Stata software
Course description
The Foundations of Clinical Research program is rooted in the belief that clinical research training is critical to professional development in health care. Clinical research training not only creates potential independent investigators, but also enables clinicians to advance their careers through a greater understanding of research evidence. Designed to provide learners with the foundational knowledge and skill sets required to produce high-quality clinical research, our program will lay the fundamental groundwork in epidemiology and biostatistics required for a multifaceted career in clinical research.
The overarching goal of the Foundations of Clinical Research program is to equip the next generation of researchers with the skill sets essential to evaluating evidence, understanding biostatistics, and beginning their clinical research careers. Our aim is to ensure that learners develop a strong foundation in the design, implementation, analysis and interpretation of clinical research studies.
During the program, our innovative active learning approach emphasizes the traditional tutorial system with weekly live video tutorials, seminars and symposia anchored by 3 live intense weekend online workshops. The Foundations of Clinical Research program’s six-month online curriculum emphasizes real-time skill-based learning.
Participants will be eligible for Associate Alumni status upon successful completion of the program. Early tuition and need-based tuition reductions may be available.
Course Outline
Live Workshops
The interactive workshop curriculum will focus on hands-on skill development through active learning. To that end, the intensive schedule is designed to accelerate the growth of high-yield clinical research skills via individual and team-based workshop exercises. Students will be immersed in a dynamic learning environment that encourages collaboration and collegial networking with faculty and peers.
Essential elements of the workshop include instruction and practical exercises in the core concepts of biostatistics, epidemiology and research question development, as well as critical assessment of the medical literature and practical training in statistical software using real-life datasets. In addition to providing training in mentorship, academic career development and leadership, we create a supportive and active learning environment where opportunities for knowledge retention and networking abound.
Live Symposia, Tutorials and Seminars
Symposia, tutorials and seminars are mandatory and will be delivered live online and organized according to eight specific clinical research topics.
Eight 3-Hour Symposia
- Instruction on a specific clinical research topic (e.g., cohort study design and interpretation)
- In-depth discussion on a related epidemiology concept (e.g., odds ratio)
- Hands-on guidance for implementing the related analysis with statistical programming in Stata
Eight 1-Hour Tutorials
- Interpret and report on papers related to the specific clinical research topic
Eight 1-Hour Special-Topic Seminars
- The biostatistical and epidemiological concepts to specific clinical research topics with concrete examples
Assignments
All students will be expected to complete all assignments by the due dates. Assignments will be graded as either “pass” or “fail.”
Individual Assignment 1
Individual Research Question and Study Design
- Generate a novel research question in the evidence-based PICO format
- Receive expert faculty review
Individual Assignment 2
Design, Implement and Present an Original Abstract
- Design and implement a clinical research study based on a publicly available dataset
- Analyze and create data visualizations via a user-friendly R Shiny web app
- Write a formal 350-word abstract suitable for submission to an international conference
- Present a digital poster to faculty at Workshop 3
Online Lectures
Research Study Introduction
- Designing a Clinical Research Study I–III
- Introduction to Evidence-Based Medicine, Systematic Review and Meta-Analysis
- Study Design 1 – Observational
- Study Design 2 – Randomized Controlled Trials
- Study Design 3 – Quasi-Experimental Studies
- Introduction to Biostatistics
- An Investigator’s Responsibility for Protection of Research Subjects
- How to Search PubMed
- Overview of Evidence-Based Medicine
Statistical Programming in Stata
- Loading Data
- Basic Programming Commands
- Data Cleansing
- Data Analytics I – Central Tendency
- Data Analytics II – Statistical Testing
- Data Analytics III – Regression Testing
Instructors

Jamie Robertson

Djøra Soeteman
You may also like.

Global Clinical Scholars Research Training
This Harvard Medical School one-year, application-based certificate program provides advanced training in health care research and methods.

Clinical Drug Development
Learning about the process of clinical drug development has important implications for anyone working in health care and related sectors.

Critical Issues in Tumor Microenvironment: Angiogenesis, Metastasis and Immunology
The 35th Annual Tumor Course reviews what is currently known about the tumor microenvironment, angiogenesis, metastasis, immunology, targeted therapies, metabolism, microbiome, and toxicity of immunotherapies.
Join our list to learn more

Clinical Research Certification
Clinical research training.
Get Clinical Research Certification with Comprehensive Clinical Trials Training Online
Get clinical research career training in 1 to 4 weeks with our online accredited clinical research courses. Trusted by organizations and experienced researchers.
Advanced ICH GCP Certification (AGCPC)
Objectives: Provide an advanced and engaging review of International Conference on Harmonization Good Clinical Practice (ICH GCP) guidelines updated for 2024.
Students Enrolled: 3,166†
Students came from: Multiple universities, hospitals, research facilities, contract research organizations, medical practices, and biopharmaceutical companies at different stages
Requirements: HS Diploma or GED
Format: Advanced, online, self-paced.
Length: 16 Hours. Online, self paced, start anytime.
Accreditation: ACCRE, Transcelerate Biopharma
Certification: Clinical research certificate online. Exam score 70% or higher on 2 attempts.
Graduates work at: University research groups, hospitals, clinics, clinical trial sites, pharmaceutical companies, and government agencies
Graduate Job Roles after course: ICH GCP clinical research training is required every 2 years for all research roles thus our graduates work in a range of fields.
Research roles: Research Assistant, Lab Assistant, Research Coordinator, Research Scholar, Postdoctoral Researcher, Graduate Research Assistant, etc. Intern roles: Research Assistant Intern, Outpatient Pharmacy Intern, Clinical roles: Clinical Affairs Intern, Clinical Fellow, Clinical Nurse, Clinical Operations Manager, Clinical Research Professional Teaching roles: Assistant Professor, Lecturer, Graduate Teaching Assistant, etc. Management roles: Clinical Research Manager, Pharmacy Operations Manager, Associate Director of Clinical Development, Vice President of Clinical Development Specialized roles: Drug Safety Associate, Regulatory Specialist, Scientific Consultant, Medical Laboratory Scientist, Pharmacovigilance Associate Other roles: NHS Primary Care QI Facilitator, Government Healthcare Recruiter, Research Ethics Coordinator (based on review of new job positions after enrollment in course)
Pharmacovigilance and Drug Safety
Advanced Pharmacovigilance and Argus Safety Certification (APVASC)
Objectives: Gain advanced education in pharmacovigilance management and proficiency in international regulatory affairs and drug safety monitoring.
Students Enrolled: 5,708†
Students came from: CROs, pharmacies, pharmaceutical companies,
Requirements: Bachelors in Biology or Natural Science OR Pharmacist Degree. Many roles require prior clinical research experience which can be gained by other entry level positions through our CRC or ICH GCP clinical research training certification.
Length: 110 Hours. Online clinical research course, self paced, start anytime.
Accreditation: ACCRE, Joint Accreditation for CE with ACPE 17.5 CME for Pharmacists.
Certification: Online certificate. Exam score 70% or higher on 2 attempts.
Graduates work at: Pharmaceutical and Biotech Companies, Healthcare Service Providers, Regulatory Bodies and Research Institutes, Consulting and Services Companies, Consumer Goods Companies, Healthcare Information and Service.
Graduate Hired As: Regulatory Affairs Associate, Clinical Trial Drug Safety Associate, Drug Safety Specialist, Patient Safety Senior Associate, Pharmacovigilance Scientist, Pharmacovigilance Manager, Senior Pharmacovigilance Associate, Pharmacovigilance Regional Head, Pharmacovigilance Analyst, Senior Director Quality, Pharmacovigilance Data Entry Manager, Principal Pharmacovigilance Scientist, QA & Medical Complaint Handling Associate, Senior Manager Medical Safety Officer, Medical Affairs Senior Scientist, Quality Manager, VP Medical Affairs, Clinical Guidelines Coordinator, Clinical Data Monitor, Pharmacovigilance Specialist, Regulatory Project Manager, Product Safety Manager, Manager Pharmacovigilance Operations, Regulatory Affairs Manager, Clinical Pharmacist Consultant, Product Vigilance Manager, Epidemiologist, Senior Manager Safety and Pharmacovigilance, Associate Director of Pharmacovigilance Department, Qualified Person Responsible for Pharmacovigilance (QPPV), Pharmacovigilance Deputy, Regulatory Affairs Manager, Senior Scientist, Senior Medical Advisor, Cosmetovigilance, Drug Safety and Medical Information Specialist, Environmental Analyst, Vice President Clinical Development, Operation Specialist (Life Cycle Safety/Drug Safety/Medical Information), Regulatory Affairs Supervisor & QPPV, Senior Director Clinical Operations, Safety, and Customer Service Excellence, Regulatory Affairs - Pharmacist, Vice President Operations, QA Executive, Health Advisor Pharmacist, COVID Health Specialist - Case and Contact Manager, Senior Project Manager, Pharmacy Manager, R&D Post-Implementation Service, Pharmacy Manager, Investigations Associate, Lead Clinical Operations Safety/Quality Responsible, Pharmacist, Trial and Supply Management, etc. (based on review of new job positions post-enrollment)
Clinical Research Associate
Advanced clinical research associate certification (acrac).
Objective: Obtain a thorough understanding of clinical research to proficiently fulfill the duties of a Clinical Research Associate.
Students Enrolled: 7,536†
Students came from: Pharmaceuticals and Biotech Companies, Clinical Research and Consulting Services, Hospitals and Healthcare Providers, Universities and Academic Institutions
Requirements: Seeking candidates with a Bachelor's in Biology/Natural Science, Nursing Degree, or MBBS/IMG Degree for entry-level positions . Consider obtaining ACRP or SOCRA credentials after gaining 2 years of experience. Students with credentials and/or 2 years of experience (18% of cohort) still choose our course to refresh their knowledge because of our comprehensive review.
Length: 200 Hours. Online, self paced, start anytime.
Accreditation: ACCRE, Joint Accreditation for CE with ACCME, ANCC, ACPE, ICPE for 17.5 CME for Physicians, Nurses, Pharmacists, and Healthcare Professionals. Candidate for Federally Qualified Post-Graduate Institution with MSA-CESS.
Graduates work at: Pharmaceutical and Biotech Companies, Clinical Research and Consulting Services, Consulting and Professional Services, Consumer Goods Companies, Hospitals and Healthcare Providers, Government Health Departments, Universities and Academic Institutions, Healthcare IT and Services.
Graduate job roles post-course: Clinical Research Associate, Clinical Trial Monitor II, Research Associate, CRA II, Scientist, Quality Assurance Analyst, Senior Clinical Research Associate, Research Associate in Discovery Immunology, Clinical Trial Monitor/CRA, Clinical Trials Project Manager, Associate Director of Research Nursing, Clinical Trial Navigator, Clinical Director for R&D, Senior Clinical Research Associate, Clinical Research Professional, Medical Science Liaison, Clinical Trial Associate III, Quality Assurance Associate II, IRB/SRC Analyst II, Project Manager, Clinical Trial Associate, Clinical Research Coordinator, Public Health Advisor, Associate Scientist II, Strategy Analyst, Clinical Research Associate II, Clinical Operations Specialist, Advisor - Development Clinical Research Scientist, Neuroscience, Associate Clinical Engineer, Clinical Trial Management Associate, Quality Supervisor, Clinical Research Data Coordinator (based on review of new job positions post-enrollment)
Clinical Research Coordinator
Advanced clinical research coordinator certification (acrcc).
Objective: Acquire comprehensive proficiency in clinical research coordinator training encompassing patient care, regulatory compliance, and trial oversight.
Students Enrolled: 3,653†
Students came from: Hospitals and Healthcare Providers:, Clinical Research and Consulting Services, Healthcare Services, Pharmaceutical and Biotech Companies, Clinical Research Centers
Requirements: HS Diploma or GED OR Nurses OR Professionals with patient experience.
Length: 150 Hours. Online, self paced, start anytime.
Accreditation: ACCRE, Joint Accreditation with ANCC for 17.5 CME for nurses.
Graduates work at: Pharmaceutical and Biotech Companies, Hospitals and Healthcare Providers, Universities and Academic Institutions, Clinical Research and Consulting Services, Diagnostic Services, Consumer Goods Companies, Healthcare Services, Cancer Treatment and Research Centers.
Graduate job roles post-course: Clinical Research Coordinator, Clinical Research Coordinator II, Lead Clinical Research Coordinator, Senior Clinical Research Coordinator, Oncology Research Coordinator, Clinical Study Coordinator, Clinical Research Data Coordinator, Clinical Research Nurse, Clinical Director/ Office Manager, Regulatory Contact/ Clinical Research Coordinator, Clinical Research/Regulatory Coordinator, Clinical Trials Specialist, Research Regulatory Specialist, Certified Clinical Research Coordinator, Clinical Research Specialist, Sr. Director of Clinical Operations (based on review of new job positions post-enrollment)
Clinical Research Assistant
Advanced Clinical Trial Assistant Certification (ACTAC)
Objective: Enhance skills required to support clinical trials, focusing on trial conduct, data collection, and administrative duties.
Students Enrolled: 1,800†
Students came from: Various universities, hospitals, clinics, and clinical research sites, etc.
Requirements: HS Diploma or GED. Current high-schoolers with evidence of active research internship can enroll.
Length: 50 Hours. Online, self paced, start anytime.
Accreditation: ACCRE, Transcelerate Biopharma.
Graduates work at: Various universities, hospitals, clinics, scholarships, internships, and clinical research sites.
Graduate job roles post-course: Clinical research assistant, clinical trial assistant, clinical researcher professional, clinical research coordinator, trial assistant, research assistant.
Clinical Research Project Manager
Advanced Clinical Research Project Manager Certification (ACRCC)
Objective: Prepare students for clinical research management certificate by teaching them how to effectively oversee large-scale clinical studies, ensuring adherence to protocols, budget, and timelines.
Students Enrolled: 1,190†
Students came from: Several CROs, universities, hospitals, clinics.
Requirements: Prior Project Management or Clinical Research Experience.
Accreditation: ACCRE, Joint Accreditation for CME
Certification: Online certificate. Exam score 70% or higher on 2 attempts.
Graduates work at: Pharmaceutical and Biotech Companies, Clinical Research and Consulting Services, Healthcare Staffing, Universities and Academic Institutions
Physician Medical Monitor
Advanced physician medical monitor certification (apmmc).
Prepare physicians for the specialized role of Medical Monitor, with an emphasis on patient safety, protocol adherence, and data interpretation.
Students Enrolled: 1,438†
Requirements: Medical Degree (MBBS, IMG, FMG).
Accreditation: ACCRE, Joint Accreditation with AMA for 17.5 CME.
Physician Principal Investigator
Advanced Principal Investigator Physician Certification (APIPC)
Equip licensed physicians in their country with the knowledge and skills to undertake the role of Principal Investigator in clinical research.
Students Enrolled: 391†
Requirements: Active Medical Degree. Nonactive medical doctors, PhDs, and PharmDs can work as Sub-I with this clinical research training certification.
Length: 100 Hours. Online, self paced, start anytime.
CCRPS Reviews : View Recent Clinical Graduate Case Studies from April 2024
From Intern at a CRO to Regulatory Affairs Specialist at UPenn
From No Prior Experience to Clinical Researcher
IMG Used CCRPS to Advance as CRC, CRA, and now research project manager
Startup Associate to a Senior Clinical Research Startup Specialist
Senior Clinical Research Professional Uses CCRPS to Advance Her Career
From Pharmacist to pharmacovigilance officer with CCRPS
Drug Safety Associate used CCRPS to Transition to American Job Market
From FMG to Clinical Research Coordinator
From Clinical Research Receptionist to Certified Study Coordinator
Transitioning from Physical Therapy to Clinical Research
From PV "student" to Lead Safety Associate with CCRPS
From Educational Research to Clinical Trials Project Management
Leaders in Online, Advanced Clinical Research Certification . Comprehensive Clinical Research Training in 1 to 4 weeks. See why 22,000+ students trust us and start or advance your clinical research career today.
Clinical research courses trusted by students at 1,200+ organizations, 6 government agencies, and 308 universities. Personalized clinical research job coaching with graduates placed at 1,600+ different organizations from large CROs to various trial sites.†(2024 CCRPS Graduate Survey).
Online, self-paced, instant Linkedin badge and online certificate after passing 70% final exam. Accredited by ACCRE, Transcelerate, Joint Accreditation (AMA, ANCC, ACPE for CME), Candidate MSA for federally-qualified post-graduate institute.
Swipe to see clinical research courses ->
Clinical Research Coordinator Certification
Course Info: Salary $59k-80k. Fee $300. Requires 2 year degree. Finish in 8 days.
Graduates work at: AstraZeneca, Sloan Kettering Cancer Center, US VA, Cedars-Sinai, Mayo Clinic, Janssen, Thermo Fisher Scientific, NewYork-Presbyterian Hospital, University of Alabama, Stony Brook Medicine, NYU Langone Health, Nova Research Institute, Quest Diagnostics, Nestle, Impact H&R, and more†.
Graduate job roles post-course: Clinical Research Coordinator, Clinical Research Coordinator II, Lead Clinical Research Coordinator, Senior Clinical Research Coordinator, Oncology Research Coordinator, Clinical Study Coordinator, Clinical Research Data Coordinator, Clinical Research Nurse, Clinical Director/Office Manager, and Regulatory Contact/Clinical Research Coordinator, and more†.
Clinical Research Associate Certification
Course Info: Salary $49k-103k+. Fee $450 (payment plans available). Requires 4 year degree. Finish in 10 days.
Graduates hired at: Moderna, Merck, IQVIA, ICON plc, Eli Lilly and Company, AstraZeneca, Deloitte, Procter & Gamble, St Jude Children's, Mount Sinai, U.S. DOH HHS, Mass General Hospital, Janssen Pharma, Memorial Sloan Kettering, Stanford University, and more.†
Graduate hired as: Clinical Research Associate, Clinical Trial Monitor II, Research Associate, CRA II, Senior Clinical Research Associate, Research Associate Immunology, Clinical Trials Project Manager, Clinical Director for R&D, Clinical Research Professional, and Clinical Trial Associate III, and more.†
Pharmacovigilance and Drug Safety Certification
Course Info: Salary $59k-140k. Fee $300. 4 year degree required. Finish in 6 days.
Graduates Hired At: Novo Nordisk, Moderna, Abbott, Accenture, CVS, FDA, VA, GoodRx, Merck, NIH, MOH Ontario, Thermo Fisher Scientific, Procter & Gamble, AbbVie, Parexel, Regeneron, Bristol Myers Squibb, IQVIA, Johns Hopkins, MD Anderson, Emory Healthcare, Eli Lilly, and more.†
Graduate Hired As: Regulatory Affairs Associate, Clinical Trial Drug Safety Associate, Drug Safety Specialist, Patient Safety Senior Associate, Pharmacovigilance Scientist, Pharmacovigilance Manager, Senior Pharmacovigilance Associate, Pharmacovigilance Regional Head, Pharmacovigilance Analyst, and Senior Director Quality, and more.
ICH GCP Certification
Course Info: Most comprehensive training available for ICH GCP. Fee $50. HS diploma required. Required every 2 years. Finish in 2 days.
Graduates hired at: FDA, NHS, Novartis, Novo Nordisk, ICON PLC, PPD, IQVIA, Parexel, Johnson & Johnson, Medtronic, UNC Health, NYU Langone Health, MD Anderson, Colorado State University, Baylor, Kaiser, Cornell, Boston University, and more†.
Graduate hired as: Research Assistant, Lab Assistant, Research Coordinator, Postdoctoral Researcher, Clinical Nurse, Clinical Operations Manager, Clinical Research Assistant Professor, Clinical Research Manager, Associate Director of Clinical Development, and Vice President of Clinical Development, and more†.
Research Project Manager Certification
Course Info: Salary $65k-143k. Fee $350. Finish in 10 days. Prior PM or Research Experience required.
Graduates work at: Emory, ION Pharmaceuticals, Inc., Baim Institute for Clinical Research, Aya Healthcare, Dermavant Sciences, Inventprise, iSTAR Medical, Oregon Health & Science University, Flinders University, and more†.
Graduates hired as: Clinical Trial Project Manager, Research Nurse Manager, Clinical Research Coordinator-Data Manager, Clinical Research Associate, Transdisciplinary Research Project Manager, IT Project Manager in Clinical Research, Publicly Funded Research Project Manager, and more†.
Medical Monitor Certification
Course Info: Salary $49k-110k. Fee $350. MBBS/IMG/MD preferred. Finish in 8 days.
Graduates hired at: US Army, Yale School of Medicine, Oxford University, Merck Healthcare, IQVIA Canada, CDC Foundation, MD Anderson Cancer and more†.
Graduates hired as: Clinical Research Medical Monitor, Medical Monitor, Principal Medical Monitor, Lead Medical Monitor, Clinical Trial Medical Monitor, Associate Medical Monitor, Senior Medical Monitor, Clinical Study Physician, Medical Advisor for Clinical Research, Clinical Research Physician, Medical Safety Monitor, Medical Director of Clinical Research, Drug Safety Medical Monitor, and more†.
Principal Investigator Certification
Course Info: Fee $300. Active MD required. Salary range 42k-112k+ not including base physician salary. Finish in 6 days (optional modules).
Graduates Hired At: Accelemed Research, Zion Healthcare, CAP Research, Quotient Sciences, and more.†
Graduates Hired As: Principal Investigator in Clinical Research, Principal Research Investigator, Senior Principal Investigator, Clinical Trial Principal Investigator, Clinical Research Nurse Investigator, Oncology Principal Investigator, Radiation Therapy Principal Investigator, Academic Principal Investigator, Healthcare Settings Principal Investigator, and more.†
Research Assistant Certification
Course Info: Salary range $25k-70k+. Fee $150. HS degree required. Finish in 6 days.
Graduates work at: Various universities, hospitals, clinics, scholarships, internships, and clinical research sites.†
Graduates hired as: Clinical research assistant, clinical trial assistant, clinical researcher professional, clinical research coordinator, trial assistant, research assistant, and more†.
Clinical Research Certification Courses
Clinical Research Associate Training
Requires bachelors of science . Monitor multiple clinical trial sites. Finish in 2-4 weeks.
Pharmacovigilance Clinical Research Training
Requires bachelors of science . Monitor drug safety. Finish in 2-3 weeks.
Clinical Research Coordinator Training
Requires 2 year degree . Support a clinical trial site. Finish in 1-3 weeks.
ICH GCP Clinical Research Training
Requires HS diploma. Required for all clinical trial professionals every 2 years .
Clinical Trial Assistant Training
Requires HS diploma . Assist in clinical trials. Finish in 1-2 weeks.
Clinical Research Management Certification Training
Requires clinical trial or project management experience . Finish in 2-4 weeks.
Principal Investigator Training
Requires active MD license or pending Sub-PI position . Conduct clinical trial at site. Finish in 1-3 weeks.
Medical Monitor Training
Requires MD or MBBS/IMG/FMG . Monitor clinical trials with medical knowledge. Finish in 2-4 weeks.
Triple Accredited Clinical Research Certification Courses for 2024-2025
Transcelerate biopharma.
Recognizes CCRPS to be an accredited GCP trainer.
CCRPS is a candidate undergoing a 1 year intensive study for approval to be a federally recognized career and technical institution.
ACCRE accredits the professional program in Clinical Research leading to the Clinical Research Certification.
Institute for Credentialing Excellence
CCRPS maintains ICE organizational membership.
Upgrade your career or switch to a new path with our online clinical research training.
Joint accreditation.
CCRPS courses accredited by ACME, ICPE, and ANCC for doctors, pharmacists, and nurses for 17.5CME.
Postgraduate Institute for Medicine
CCRPS is audited by PIM for CME credit approval.
About CCRPS
Our online program for clinical research certification is trusted by thousands of students with our graduates finding careers at over 1,600 companies after taking our course per our 2024 survey. Ideal for career changers or those wanting to advance in roles like clinical research associate, coordinator, assistant, project manager, drug safety officer, principal investigator, or medical monitor.
Clinical research training courses by CCRPS are accredited by major organizations (ACCRE, Transcelerate Biopharma, AMA, ACPE, ANCC, ICPE for CME through JA) and recognized by small to large-size clinical research organizations.
Developed by senior clinical research training professionals to help students of all levels.
Training for a New Generation of Researchers
CCRPS provides affordable, industry-recognized clinical research training that will improve your job prospects and trial outcomes. We offer ICH GCP training, CRA certification, CRC certification, research assistant training, pharmacovigilance certification, PI training, medical monitor, and clinical research project manager training. We serve clinical professionals including nurses, physicians, pharmacists, PhDs, premeds, and science-field graduates who want to transition or accelerate their careers with CCRPS.
Do you want more information on our selection of clinical research training certification online programs? Read below.
Clinical Research Courses
The ICH-GCP certification is out for 2024 and offers hours worth of in depth clinical research certification training on all aspects about Good Clinical Practice as defined by the International Conference on Harmonization. The most advanced modules provide a complete overview no matter what your background with pharmaceutical research might be; this includes ethical practices that prioritize safety along side transparent decision making processes where there are none!
Requirements for ICH GCP Certification
The only diplomas needed to enroll in this program are high school or equivalent level education (such as GED). However, if you have more training than that and would like a head start on understanding the material being taught at our college then it is recommended that prior learning be taken into consideration when scheduling classes.
Is ICH GCP Certification right for you?
The ICH-GCP clinical research certification training is a great way for anyone who needs an introduction or refresh on ICH GCP guidelines in order to be an great CCRP. Candidates appearing before interviewers may find themselves unprepared when it comes down solely and exclusively them, but this course will give you all the basics that are needed!
Download the ICH GCP guidelines .
Why choose our ICH GCP training?
ICH-GCP clinical research certification training online confers multiple benefits not matched by any other GCP certification currently available, including being E6 (R2) compliant, having instant enrollment and flexible scheduling, being industry recognized, accredited by research authorities, and affordable with flexible payment options.
Institutions such as CROs that require employees to complete GCP certification can opt for a one-time annual fee payment which allows flexible scheduling for an unlimited number of trainees.
Research Assistant - Clinical Research Assistant
The research assistant certification provides you with the kick-start that will help gain better visibility for your application. The course is designed give thorough understanding of criteria needed in order conduct them effectively, what makes one organization more desirable than another when it comes time apply. The modules cover all aspects from planning through documentation, reporting & publication as well as safety practices necessary during participant recruitment/screening procedures
Requirements for Research Assistant Certification
The research assistant training is open to anyone, even without a high school diploma or equivalent.
High school students intending to work after graduation or interested in healthcare research may benefit from completing the clinical research assistant certification.
Premed students enrolled in an undergraduate degree program and majoring in one of the life sciences may also benefit from clinical research assistant training.
Why choose our clinical research assistant certification?
The research assistant certification is the leading choice for research assistant jobs because it is fully compliant with ICH-GCP and FDA CFR, covers all key concepts extensively, has flexible scheduling, is widely recognized and accepted, and is affordable.
Is research assistant training right for you?
The research assistant program provides a strong understanding of advanced Good Clinical Practice to have a successful career with room for growth. This program offers hands-on experience in subject-facing dimensions of clinical research trials, including training in: the proper protocol for obtaining informed consent, eliciting subject cooperation during trials, obtaining necessary background information for trial documentation.
Trainees learn about subject safety monitoring, which includes exposure to: Adverse Event (AE) identification, documentation and reporting, and trial protocol adherence.
The research assistant course materials contain real-life examples and case studies to help trainees develop insight into and build strategies for: increasing subject enrollment in new and ongoing studies, improving retention rates among subjects enrolled in an ongoing study.
This advanced clinical research coordinator training program is designed to provide in-depth coverage of all aspects, from basic pharmacovigilance and regulatory audits right up through planning for scientific integrity. The course teaches students everything they need know about how best handle each situation that may arise during their career as a Clinical Research Coordinator - no matter what field area interests them most!
Requirements for CRC Certification
The clinical research coordinator (CRC) is a senior member of the clinical research team with responsibilities in overseeing the smooth conduct of clinical research. Candidates must possess a minimum of an associates degree.
Is clinical research coordinator training right for you?
The objectives of candidates enrolling in the clinical research coordinator course are typically related to advancing their clinical research careers. Research professionals enroll in the program to build the relevant knowledge base and administrative skills needed to strengthen their applications for CRC positions. Use the clinical research coordinator course refresh or upgrade their skill-set and obtain clinical research certification in coordination.
Why choose our CRC Certification?
Our clinical research coordinator training has emerged as the clear industry preference when it comes to certifying candidates for on-site roles in clinical research, due to its updated compliance information, broad and deep content coverage, flexible scheduling, and industry-wide reputation for quality.
Our clinical research coordinator certification is accredited by the ACCRE, ACCME, ACPE and ANCC - the most widely recognized and accepted CRC programs across the industry - making it a sound investment for those looking to pursue a career in clinical research.
The course tuition is affordable and can be paid up-front or in easy monthly installments
The Clinical Research Associate Program is the perfect opportunity for you to have a career in research! This advanced program has over 200 hours of specialized clinical research training, which will teach students everything they need. You'll learn how to write reports and site visits with ease using our curriculum that covers all topics related directly or indirectly toward clinical trials work--and even teaches additional techniques for efficiency and workflow.
Requirement for CRA Certification
In order to enroll for the clinical research associate certification , one must have a bachelor’s degree in life science or a health-care science, or a graduate degree in medicine.
Is CRA training right for you?
Graduates with a bachelor's degree in science who are interested in exploring clinical research can benefit from taking this course. Aspirants to CRA jobs looking to boost their hire visibility can also benefit from taking the course. Health-care professionals (RNs, NPs, PAs and others) aiming to either transition to or advance a career in clinical research.
CRAs with less than 5 years of work experience wishing to fast-track. Also, CRAs, Senior CRAs and other clinical research personnel needing a refresher course.
Why choose our CRA Certification?
The clinical research associate course confers a number of advantages on the individual, whether they are entering the field of clinical research or working to advance their career.
CRAs certified through clinical research associate training have up-to-date knowledge of both ICH and FDA regulatory requirements for human subject safety in clinical research.
The program is flexible, allowing trainees to fit the training into a busy schedule. There is an emphasis on hands-on clinical research training using real-life clinical research examples and data sets.
Completing the clinical research associate course is recognized across the US as equivalent to 17.5 CME credits.
Qualifying candidates receive not only a widely accepted and recognized clinical research associate certificate.
Pharmacovigilance
The pharmacovigilance course is an advanced program that will prepare you for a career in PV, with the most comprehensive syllabus covering all aspects from pre-clinical phase to post market surveillance (Phase IV clinical trials).
Requirements for Pharmacovigilance Clinical Research Certification
The goal of pharmacovigilance is to ensure the safety of all drugs and medical devices. QPPVs are responsible for achieving this goal through and beyond clinical trials. To be a QPPV, one must have considerable medical knowledge, statistical skill, and analytical ability. Candidates for the pharmacovigilance and regulatory affairs certification must possess a minimum of:
A bachelor’s degree in life science OR a health-care science
Is clinical research drug safety certification right for you?
The pharmacovigilance certification is beneficial for those in the clinical research field who wish to upgrade their qualifications and expertise. CTAs/CRAs, SCRAs/CRCs, medical and nursing professionals, and QPPVs can all benefit from enrolling in the program.
Enrolling in the pharmacovigilance training gives aspirants an edge when applying for positions that require advanced knowledge of PV compliance, data analytics, software management skills, medical-legal awareness, etc.
Why choose our pharmacovigilance and drug safety training?
Our drug safety and regulatory affairs course is one of the leading pharmacovigilance clinical research certification programs by recruiters across the industry. The pharmacovigilance clinical research certification is compliant with FDA CFR and WHO-ISoP, providing trainees with up-to-date coaching on all relevant regulatory codes and standards. The focus areas of the pharmacovigilance course comprehensively cover all domains of knowledge and skill required for an effective QPPV. The pharmacovigilance course trains candidates in creating, managing and retrieving case report forms using Argus Safety software. CCRPS regulatory affairs clinical research certification offers on-demand, flexible scheduling to allow enrolled students to complete the program at their own pace. The pharmacovigilance and drug safety course tuition is payable either up front or in two easy monthly installments.Explore comprehensive clinical data management training and placement opportunities in the USA. Develop your skills and secure promising positions in this dynamic field. Unlock your potential for success today.
How to Become A Trial Project Manager
Requirements for clinical trial project manager training
Clinical research project managers must have a bachelor's degree in a scientific field. We require prior clinical trial experience in managerial roles or prior project manager experience though graduates seeking to grow in their current career can take the course. They must be able to manage and coordinate all aspects of clinical trials. They must be able to keep up with ever-changing regulations governing clinical trials
Why choose our medical monitor training?
Our clinical trial project manager training is the most comprehensive and up-to-date clinical research certificate program online. You will learn how to manage clinical trials from start to finish, including budgeting, scheduling, and communication.
Upon completion of the program, you will be a certified clinical trial project manager . Our tuition rates are very affordable compared to other programs in this field.
Is project manager clinical research certification right for you?
If you're a project manager or coordinator who is looking to enhance your skills and salary, then clinical research project manager training may be right for you. Earning clinical research project manager certification can help you stand out from other project managers and improve your career prospects.
You must also pass an exam that covers topics such as risk management, stakeholder relationships and data management.
If you meet these qualifications, then becoming certified can help you demonstrate your knowledge and expertise in the field of clinical research project management . Not only will this make you a more valuable asset to your current employer, but it can also open up doors to new opportunities down the road.
Certified clinical research professionals work in a booming industry and there’s no doubt that project managers are in high demand. If you want to make the jump into clinical trial project management, or if you’re already a project manager but want to specialize in pharmaceuticals, our course is exactly what you need.
How to Become a Medical Monitor
Requirements for medical monitor training
Medical monitors are senior members of the clinical research team who oversee the ethical, safe, and transparent conduct of clinical research.
To qualify as a medical monitor, trainees must have a degree in medicine (MD), a non-US degree in medicine (IMG/FMG), or a master’s degree in pharmacy (PharmD).
Physicians with one or more years of exposure to medical research may also qualify as medical monitors.
The medical monitor certification is a program that covers the full range of knowledge domains essential for an medical monitor role, from the philosophy behind GCP to present-day regulatory requirements for clinical research. The course curriculum reflects the most updated regulatory policies related to FDA’s CFR Title 21, as well as E6 (R2) ICH-GCP guidelines. Trainees have the option of on-demand scheduling to fit with their busy schedules.
Is Medical monitor certification right for you?
The medical monitor training offers a comprehensive overview of the principles of Good Clinical Practice, as well as compliance requirements for ethical and safe medical research.
This is the only program that provides in-depth training on all aspects of clinical research design and execution.
The medical monitor course also covers pharmacovigilance concepts crucial to a medical monitor’s role such as AE/SAE identification and tracking; probabilistic assessment of AEs/SAEs as ADRs; risk management in clinical trials.
Trainees gain working knowledge of financial regulatory compliance: disclosure documentation & updating; FDA audit protocols & strategies.
An added advantage is its focus on digitized elements such remote data monitoring tools (software & video), EDC capture & quality control
Principal Investigator
The principal investigator certification program is a great way for physicians involved in clinical research who want to transition into more senior roles, enhance their eligibility when applying or overseeing trials process. It provides PIs with the ideal means of upgrading career skills while also helping them become better fundraisers and managers!
Requirements for Principal Investigator Certification
To be a certified PI, you must be a practicing physician. You may also either be the PI or Co-PI of an ongoing clinical research study, or have been the Ex-PI or former Co-PI of a completed study.
Is Principal Investigator training right for you?
The principal investigator certification provides a thorough, yet quick refresher of the regulatory and compliance requirements for ethical, safe and transparent medical research. This is an in-depth review of all aspects of leading clinical research design and execution as a principal investigator, including: advanced trial design, randomization, blinding and unblinding; clinical site assessment, preparation active site monitoring and close-out; clinical trial protocol development and implementation, including trial monitoring tools and documentation; Investigational Product (IP) accountability storage and dispensing; Adverse Events (AEs), Serious Adverse Events (SAEs), Adverse Drug Reaction (ADR), Important Medical Event (IME) – identification tracking reporting; probabilistic assessment of AEs SAEs as ADRs – medical assessment statistical data analytics risk safety assessments in clinical trials.
Why choose our PI training course?
The principal investigator certification is the best choice for both physicians who want to get certified as a PI, and for industry experts who are looking for someone to fill a PI position. This is because the certification is very flexible and covers a lot of ground.
Additionally, those who become certified principal investigators will be up-to-date on the most recent regulatory policies related to FDA CFR Title 21 and the E6 (R2) ICH-GCP guidelines. This means that they will be qualified to manage compliance requirements in a clinical study.
The course curriculum includes all of the knowledge domains essential for clinical research principal investigator training , but busy professionals can review only the modules most relevant to them and their needs. This way, they can still update their knowledge and skills without having to spend a lot of time on it.
Clinical Research Staff Training
CCRPS works with pharmaceutical, biotech, medical device, and contract research organizations to efficiently train and certify their clinical research associates, coordinators, and assistants to meet ICH GCP and CFR compliance for their staff. We can provide outsourced clinical research staff training set up within 1-2 business days. We work with organization budgets and staff training size to provide comprehensive and transformational education for onboarding and updating staff compliance with ICH GCP and job training requirements. We have worked directly with organizations and groups ranging from 2 employees to 179 employees.
The Platform for Clinical Research Education
Ccrps case studies & reviews.
From IMG to Clinical Research Coordinator at Columbia University: " This course not only met but exceeded my expectations with its thorough curriculum and insightful modules." -Lisa-Pierre ( view full case study )
From IMG to Clinical Research Coordinator "The hands-on activities integrated throughout the course really helped solidify my understanding of complex concepts." -Umber Mahmood ( case study summary )
From Physical Therapist to Clinical Researcher: "The in-depth content and expert instructors provided me with invaluable insights into the field." - Celia Moon ( case study summary )
From International CRC to U.S. Lead CRC and CRA: "The flexible online format allowed me to balance my studies with my professional commitments seamlessly." - Aishwarya Sukumar ( view full case study )
Enjoyed Clinical Research Training through Examples "The real-world examples used throughout the course were incredibly useful for applying theory to practice." -Marta Marszalek ( view full case study )
Promoted to Senior Startup Specialist in Clinical Trials : "I appreciate how the course was structured—very interactive and engaging from start to finish." -Justin Scott Brathwaite ( transcript summary )
From Clinical Research Receptionist to Certified Study Coordinator with CCRPS: "I highly recommend this course for its comprehensive approach and practical applications." - Katie Decker ( view full case study )
Learning to Lead Safety Associate: "The course materials were clear, well-organized, and directly applicable to my work." - Renata Noronha ( view full case study )
From IMG to securing roles as a CRC, CRA, and now a project manager: "Joining this course was a pivotal step in my career advancement." - Dr. Vrushali Borawak ( view full case study )
From Physician to Confident Drug Safety Specialist: "The course provided a robust foundation in the field, which was critical for my professional development." - Rabiea Bilal ( view full case study )
From plant biologist to clinical recruitment administrative coordinator : "This program is a gateway to extensive knowledge and skills in a supportive learning environment." -Olajumoke Owati ( view full case study )
ICH GCP Expert: "Thanks to this course, I feel more competent and confident in my role." - Stephanie ( case study summary )
From International PV Roles To North American Market Success: "The detailed modules prepared me excellently for real-world applications." - John Vinil ( view full case study )
From Educational Research to Clinical Trials Project Management: "I was able to immediately apply what I learned in the course to my job. " - Rose Hyson ( view full case study )
From Coordinator to Clinical Research Grant Manager: "it really did a great job of the full scope of clinical research from start to finish." -Hannah Fischer
ICH GCP made her more confident in research: "this course just overall did a really good job going in depth, which I feel like wasn't just, it wasn't just covered just for the sake of covering content" - Aastha Shah (view full transcript)
From Clinical Research Intern to Regulatory Affairs Associate at UPenn: "I would say since then. I've completed this course. It's helped me get my job in regulatory affairs at a clinical research site." - Scott Boyle
From Masters in Health Safety to Clinical Researcher: " I will say quality of delivery, quality of the materials. - Ossai Opene ( view full case study )
- Biochemistry and Molecular Biology
- Biostatistics
- Environmental Health and Engineering
- Epidemiology
- Health Policy and Management
- Health, Behavior and Society
- International Health
- Mental Health
- Molecular Microbiology and Immunology
- Population, Family and Reproductive Health
- Program Finder
- Admissions Services
- Course Directory
- Academic Calendar
- Hybrid Campus
- Lecture Series
- Convocation
- Strategy and Development
- Implementation and Impact
- Integrity and Oversight
- In the School
- In the Field
- In Baltimore
- Resources for Practitioners
- Articles & News Releases
- In The News
- Statements & Announcements
- At a Glance
- Student Life
- Strategic Priorities
- Inclusion, Diversity, Anti-Racism, and Equity (IDARE)
- What is Public Health?
- Clinical Trials Certificate Program
Sponsored By: Department of Epidemiology
Onsite or Online | Part-Time | 1-3 years
- MSPH Field Placements
- Master's Essays
- MAS Application Fee Waiver Requirements
- Master of Arts and Master of Science in Public Health (MA/MSPH)
- Master of Arts in Public Health Biology (MAPHB)
- Master of Bioethics (MBE)
- Mission, Vision, and Values
- Student Experience
- Program Outcomes
- For Hopkins Undergraduate Students
- Master of Health Science (MHS) - Department of Biochemistry and Molecular Biology
- Master of Health Science (MHS) - Department of Epidemiology
- Alumni Update
- MHS Combined with a Certificate Program
- Master of Health Science (MHS) - Department of Molecular Microbiology and Immunology
- Bachelor's/MHS in Health Economics and Outcomes Research
- MHS HEOR Careers
- Frequently Asked Questions
- Master of Health Science (MHS)
- Concurrent School-Wide Master of Health Science Program in Biostatistics
- Master of Health Science - Department of Population, Family and Reproductive Health
- Master of Health Science Online (MHS) - Department of Population, Family and Reproductive Health
- Careers in Health Economics
- Core Competencies
- Meet the Director
- What is Health Economics
- MPH Capstone Schedule
- Concentrations
- Online/Part-Time Format
- Requirements
- Tuition and Funding
- Executive Board Faculty
- Master of Science (ScM) - Department of Biochemistry and Molecular Biology
- Master of Science (ScM) - Department of Biostatistics
- Master of Science (ScM) - Department of Epidemiology
- Master of Science (ScM) - Department of Molecular Microbiology and Immunology
- Bachelor's/MSPH in Health Policy
- FAQ for MSPH in Health Policy
- Field Placement Experience
- MSPH Capstone
- MSPH Practicum
- Required and Elective Courses
- Student Timeline
- Career Opportunities
- 38-Week Dietetics Practicum
- Completion Requirements
- MSPH/RD Program FAQ
- Program Goals
- Application Fee Waiver Requirements
- Doctor of Philosophy (PhD) - Department of Biostatistics
- Doctor of Philosophy (PhD) - Department of Epidemiology
- Program Goals and Expectations
- Doctor of Philosophy (PhD) - Department of Molecular Microbiology and Immunology
- Doctor of Philosophy (PhD) - Department of Population, Family and Reproductive Health
- Doctor of Philosophy (PhD) in Clinical Investigation
- Recent Graduates and Dissertation Titles
- PhD Funding
- PhD TA Requirement
- Recent Dissertation Titles
- JHU-Tsinghua Doctor of Public Health
- Prerequisites
- Concentration in Women’s and Reproductive Health
- Custom Track
- Concentration in Environmental Health
- Concentration in Global Health: Policy and Evaluation
- Concentration in Health Equity and Social Justice
- Concentration in Health Policy and Management
- Concentration in Implementation Science
- Combined Bachelor's / Master's Programs
- Concurrent MHS Option for BSPH Doctoral Students
- Concurrent MSPH Option for JHSPH Doctoral students
- Doctor of Medicine and Doctor of Philosophy (MD/PhD)
- Adolescent Health Certificate Program
- Bioethics Certificate Program
- Community- Based Public Health Certificate Program
- Demographic Methods Certificate Program
- Epidemiology for Public Health Professionals Certificate Program
- Evaluation: International Health Programs Certificate Program
- Frequently Asked Questions for Certificate Programs
- Gender and Health Certificate Program
- Gerontology Certificate Program
- Global Digital Health Certificate Program
- Global Health Certificate Program
- Global Health Practice Certificate Program
- Health Communication Certificate Program
- Health Disparities and Health Inequality Certificate Program
- Health Education Certificate Program
- Health Finance and Management Certificate Program
- Health and Human Rights Certificate Program
- Healthcare Epidemiology and Infection Prevention and Control Certificate Program
- Humanitarian Health Certificate Program
- Implementation Science and Research Practice Certificate Program
- Injury and Violence Prevention Certificate Program
- International Healthcare Management and Leadership Certificate Program
- Leadership for Public Health and Healthcare Certificate Program
- Lesbian, Gay, Bisexual, Transgender, and Queer (LGBTQ) Public Health Certificate Program
- Maternal and Child Health Certificate Program
- Mental Health Policy, Economics and Services Certificate Program
- Non-Degree Students General Admissions Info
- Pharmacoepidemiology and Drug Safety Certificate Program
- Population Health Management Certificate Program
- Population and Health Certificate Program
- Public Health Advocacy Certificate Program
- Public Health Economics Certificate Program
- Public Health Informatics Certificate Program
- Public Health Practice Certificate Program
- Public Health Training Certificate for American Indian Health Professionals
- Public Mental Health Research Certificate Program
- Quality, Patient Safety and Outcomes Research Certificate Program
- Quantitative Methods in Public Health Certificate Program
- Requirements for Successful Completion of a Certificate Program
- Rigor, Reproducibility, and Responsibility in Scientific Practice Certificate Program
- Risk Sciences and Public Policy Certificate Program
- Spatial Analysis for Public Health Certificate Program
- Training Certificate in Public Health
- Tropical Medicine Certificate Program
- Tuition for Certificate Programs
- Vaccine Science and Policy Certificate Program
- Online Student Experience
- MAS and Affiliated Certificate Programs
- Barcelona Information
- Registration, Tuition, and Fees
- Agency Scholarship Application
- General Scholarship Application
- UPF Scholarship Application
- Course Evaluations
- Online Courses
- Registration
- General Institute Tuition Information
- International Students
- Directions to the Bloomberg School
- All Courses
- Important Guidance for ONSITE Students
- D.C. Courses
- Registration and Fees
- Cancellation and Closure Policies
- Application Procedures
- Career Search
- Current Activities
- Current Trainees
- Related Links
- Process for Appointing Postdoctoral Fellows
- Message from the Director
- Program Details
- Admissions FAQ
- Current Residents
- Elective Opportunities for Visiting Trainees
- What is Occupational and Environmental Medicine?
- Admissions Info
- Graduates by Year
- Compensation and Benefits
- How to Apply
- Academic Committee
- Course Details and Registration
- Tuition and Fees
- ONLINE SOCI PROGRAM
- Principal Faculty
- General Application
- JHHS Application
- Our Faculty
- Descripción los Cursos
- Programa en Epidemiología para Gestores de Salud, Basado en Internet
- Consultants
- Britt Dahlberg, PhD
- Joke Bradt, PhD, MT-BC
- Mark R. Luborsky, PhD
- Marsha Wittink, PhD
- Rebekka Lee, ScD
- Su Yeon Lee-Tauler, PhD
- Theresa Hoeft, PhD
- Vicki L. Plano Clark, PhD
- Program Retreat
- Mixed Methods Applications: Illustrations
- Announcements
- 2023 Call for Applications
- Jennifer I Manuel, PhD, MSW
- Joke Bradt, PhD
- Josiemer Mattei, PhD, MPH
- Justin Sanders, MD, MSc
- Linda Charmaran, PhD
- Nao Hagiwara, PhD
- Nynikka R. A. Palmer, DrPH, MPH
- Olayinka O. Shiyanbola, BPharm, PhD
- Sarah Ronis, MD, MPH
- Susan D. Brown, PhD
- Tara Lagu, MD, MPH
- Theresa Hoft, PhD
- Wynne E. Norton, PhD
- Yvonne Mensa-Wilmot, PhD, MPH
- A. Susana Ramírez, PhD, MPH
- Animesh Sabnis, MD, MSHS
- Autumn Kieber-Emmons, MD, MPH
- Benjamin Han, MD, MPH
- Brooke A. Levandowski, PhD, MPA
- Camille R. Quinn, PhD, AM, LCSW
- Justine Wu, MD, MPH
- Kelly Aschbrenner, PhD
- Kim N. Danforth, ScD, MPH
- Loreto Leiva, PhD
- Marie Brault, PhD
- Mary E. Cooley, PhD, RN, FAAN
- Meganne K. Masko, PhD, MT-BC/L
- PhuongThao D. Le, PhD, MPH
- Rebecca Lobb, ScD, MPH
- Allegra R. Gordon, ScD MPH
- Anita Misra-Hebert, MD MPH FACP
- Arden M. Morris, MD, MPH
- Caroline Silva, PhD
- Danielle Davidov, PhD
- Hans Oh, PhD
- J. Nicholas Dionne-Odom, PhD RN ACHPN
- Jacqueline Mogle, PhD
- Jammie Hopkins, DrPH, MS
- Joe Glass, PhD MSW
- Karen Whiteman, PhD MSW
- Katie Schultz, PhD MSW
- Rose Molina, MD
- Uriyoán Colón-Ramos, ScD MPA
- Andrew Riley, PhD
- Byron J. Powell, PhD, LCSW
- Carrie Nieman MD, MPH
- Charles R. Rogers, PhD, MPH, MS, CHES®
- Emily E. Haroz, PhD
- Jennifer Tsui, Ph.D., M.P.H.
- Jessica Magidson, PhD
- Katherine Sanchez, PhD, LCSW
- Kelly Doran, MD, MHS
- Kiara Alvarez, PhD
- LaPrincess C. Brewer, MD, MPH
- Melissa Radey, PhD, MA, MSSW
- Sophia L. Johnson, PharmD, MPH, PhD
- Supriya Gupta Mohile, MD, MS
- Virginia McKay, PhD
- Andrew Cohen, MD, PhD
- Angela Chen, PhD, PMHNP-BC, RN
- Christopher Salas-Wright, PhD, MSW
- Eliza Park MD, MS
- Jaime M. Hughes, PhD, MPH, MSW
- Johanne Eliacin, PhD, HSPP
- Lingrui Liu ScD MS
- Meaghan Kennedy, MD
- Nicole Stadnick, PhD, MPH
- Paula Aristizabal, MD
- Radhika Sundararajan, MD
- Sara Mamo, AuD, PhD
- Tullika Garg, MD MPH FACS
- Allison Magnuson, DO
- Ariel Williamson PhD, DBSM
- Benita Bamgbade, PharmD, PhD
- Christopher Woodrell MD
- Hung-Jui (Ray) Tan, MD, MSHPM
- Jasmine Abrams, PhD
- Jose Alejandro Rauh-Hain, MD
- Karen Flórez, DrPH, MPH
- Lavanya Vasudevan, PhD, MPH, CPH
- Maria Garcia, MD, MPH
- Robert Brady, PhD
- Saria Hassan, MD
- Scherezade Mama, DrPH
- Yuan Lu, ScD
- 2021 Scholars
- Sign Up for Our Email List
- Workforce Training
- Cells-to-Society Courses
- Course/Section Numbers Explained
- Pathway Program with Goucher College
- The George G. Graham Lecture
About the Clinical Trials Certificate Program
Clinical Trials are a key tool in the evaluation of new strategies for prevention and treatment of disease. This certificate program focuses primarily on the design and analysis of randomized clinical trials for evaluation of licensed and non-licensed medical products and other health interventions, the regulatory framework for the conduct and evaluation of data from clinical trials, and ethical principles for the conduct of clinical trials.
Educational Objectives
After completing the certificate, students will be able to:
- Summarize the history of clinical trials and describe the role they play in evaluation of health interventions;
- Explain the key differences, advantages and disadvantages of experimental versus observational study designs;
- Develop a protocol, consent statement, monitoring plan and data collection plan for a clinical trial;
- Review and critique manuscripts presenting the results of clinical trials using CONSORT guidelines; and
- Explain the key ethical principles regarding the design, conduct and analysis of clinical trials
Curriculum for the Clinical Trials Certificate Program
Please visit our Academic Catalogue to see the full certificate curriculum requirements. Please also review the certificate completion requirements .
Admissions Requirements
Degree students.
The certificate program is open to graduate students currently enrolled in any division of the Johns Hopkins University, with the exception of MAS students, who are not eligible to apply until they have completed their primary degree program.
Applying to the certificate program as a JHU graduate student:
Applicants who are already enrolled in graduate programs at JHU must submit a short letter of interest and CV/resumé to the Certificate Program Contact, and complete a Declaration of Intent form prior to starting coursework.
Eligible Start Terms :
Email Certificate Program Contact
Non-Degree Students
Students with at least a baccalaureate degree from an accredited college or university and a strong academic record are eligible for admission to this certificate program.
1st and 3rd
Applying to the certificate program as a non-degree student:
Applicants who are not currently enrolled in a graduate program at JHU are required to apply to certificate programs using SOPHAS Express . All non-degree applicants should review the general Certificates Admissions page for instructions on how to apply to a certificate program.
Prerequisites or special requirements
Application for Non-Degree Students
Information regarding the cost of tuition and fees can be found on the Bloomberg School's Certificate Programs Tuition page .
Financial Aid Eligibility: U.S. citizens and U.S. permanent residents enrolled in this certificate program may be eligible to apply for Title IV financial aid. Please contact the JHU Office of Student Enrollment and Account Management (SEAM) for more information.
Questions about the program? We're happy to help.
Sponsoring Departments Epidemiology
Certificate Program Contact Sheila Small [email protected]
Faculty Sponsor Ann-Margret Ervin
- NIH Employee Intranet
- Staff Directory
- En Español
OCRECO Home > Clinical Research Education > Introduction to the Principles and Practice of Clinical Research (IPPCR)
OFFICE OF CLINICAL RESEARCH EDUCATION AND COLLABORATION OUTREACH
- Clinical Research Education
- Funding Opportunities

Introduction to the Principles and Practice of Clinical Research (IPPCR)
- Course Information
- Registration
- Course Login
The Introduction to the Principles and Practice of Clinical Research (IPPCR) course trains participants on how to effectively and safely conduct clinical research. The course focuses on the spectrum of clinical research and the research process by highlighting biostatistical and epidemiologic methods, study design, protocol preparation, patient monitoring, quality assurance, ethical and legal issues, and much more. This course will be of interest to physicians, scientists, medical and dental students, nurses, public health professionals, and others conducting or planning a career in clinical research.
Course Objectives
Provide an overview of basic biostatistical and epidemiologic methods involved in conducting clinical research.
Describe the principles involved in the ethical, legal, and regulatory issues in clinical human subjects research, including the role of Institutional Review Boards (IRBs).
Describe principles and issues involved in monitoring patient-oriented research.
Describe the infrastructure required in performing clinical research and the steps involved in developing and funding research studies.
General Information

This online course is offered annually at no cost. After registering, participants may begin their study immediately.
There are approximately 40 pre-recorded lectures (15-90 minutes each), lecture materials, templates, and discussion boards to engage with other participants and the faculty.
There is an online final exam, which consists of 50-80 multiple choice questions. Participants are permitted to use course materials during the final exam.
A Certificate of Completion is awarded to each registered participant who achieves a score of 80% or higher on the final exam.
There is no academic credit or continuing medical education (CME) credit offered for this course.
Important Dates
| Registration | September 3, 2024 – June 27, 2025 (12PM US ET) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Course Access |
Supplemental MaterialsThe course textbook, Principles and Practice of Clinical Research, Fourth Edition (2018, ISBN: 978 0-12-849905-4) is available for purchase from several online book retailers and at the NIH Building 10 FAES book store. Copies of the textbook can be purchased through Elsevier with a 30% discount and free shipping using the discount code BIOMED30 . Course Directors
Get IPPCR Course Updates Subscribe Social Media Links
Page Footer
National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892 NIH…Turning Discovery Into Health® 100 Most Popular Courses For September Harvard and MIT’s $800 Million Mistake: The Triple Failure of 2U, edX, and Axim CollaborativeThe future of Coursera’s only credible alternative for universities rests in the hands of 2U’s creditors.
600 Free Google Certifications Most commonPopular subjects. Computer Networking Digital Marketing Artificial Intelligence Popular coursesA Life of Happiness and Fulfillment Arab-Islamic History: From Tribes to Empires Inglés empresarial: ventas, gestión y liderazgo Organize and share your learning with Class Central Lists. View our Lists Showcase Clinical Research Courses and CertificationsLearn Clinical Research, earn certificates with paid and free online courses from Harvard, Stanford, University of Michigan, Johns Hopkins and other top universities around the world. Read reviews to decide if a class is right for you.
Understanding Clinical Research: Behind the StatisticsIf you’ve ever skipped over`the results section of a medical paper because terms like “confidence interval” or “p-value” go over your head, then you’re in the right place.
Improving Healthcare Through Clinical ResearchOn this free online course, find out how medical treatments are discovered, tested and evaluated to improve healthcare for all.
What is Health Research?Explore the world of health research and the role volunteers play in transforming treatments and improving health care.
Current Regulatory Requirements for Conducting Clinical Trials in IndiaComprehensive overview of India's clinical trial regulations, covering key aspects and requirements for conducting ethical and compliant research in the country.
Thinking Critically: Interpreting Randomized Clinical TrialsEnhance critical appraisal skills for evaluating clinical research papers through engaging videos and practical examples. Improve clinical practice, research quality, and peer-review abilities.
Design and Interpretation of Clinical TrialsLearn to design, conduct, and interpret clinical trials for evaluating medical interventions. Explore trial designs, randomization, masking, outcome selection, analysis methods, ethical considerations, and reporting standards. Translating Research to PatientsExplore clinical trials, ethical research practices, and regulatory processes in translating scientific discoveries to patient care. Gain insights into human subjects research and its role in advancing medical knowledge.
Clinical Trials OperationsDevelop essential skills for designing, managing, and analyzing clinical trials, covering ethics, safety, recruitment, compliance, and reporting standards in healthcare research.
Design and Conduct of Clinical TrialsComprehensive guide to designing and conducting clinical trials, covering bias control, trial stages, outcome definition, ethical considerations, and participant recruitment and retention strategies.
Clinical Trials Data Management and Quality AssuranceLearn to manage clinical trial data, ensure quality, and maintain integrity through effective collection, organization, monitoring, and quality assurance practices for successful research outcomes.
Clinical Trials Management and Advanced OperationsExplore advanced clinical trial management, covering protocol events, regulatory compliance, standardization, transparency, evidence synthesis, and essential study documentation for effective research conduct.
Clinical Trials Analysis, Monitoring, and PresentationLearn advanced skills for clinical trial management, including sample size calculation, statistical monitoring, result reporting, and the analyst's role throughout the process.
Data Management for Clinical ResearchLearn critical concepts and practical methods for planning, collecting, storing, and disseminating data in clinical research. Covers electronic data capture, regulatory compliance, and best practices for effective data management.
Partnering with the Public and Patients in Medical ResearchExplore patient-researcher partnerships in medical research, learning strategies for inclusivity, trust-building, and mutual benefit to enhance research outcomes and patient empowerment.
Advanced Knowledge and Skills of Originator and Biosimilar Biologics: For Health Care ProvidersDeveloped by leading researchers and clinicians from across Canada, this course will enhance knowledge and skills for clinicians who are using or beginning to use a biosimilar or biologic medicine in their professional practice setting.
Never Stop Learning.Get personalized course recommendations, track subjects and courses with reminders, and more.  Clinical Research, Graduate CertificateQuicklinks: Curriculum • Cost Program at a Glance
Clinical research is the fastest-growing discipline in the burgeoning medical device and pharmaceutical industries. Keeping pace with the ever-increasing body of knowledge and new regulatory procedures generated by advances in clinical research is critical to career success. Boston University’s online Graduate Certificate in Clinical Research—designed and offered by BU’s School of Medicine—is an opportunity for clinical researchers, health professionals, and those interested in clinical research to gain an in-depth understanding of the scientific fundamentals of human research. Courses in the curriculum provide an in-depth look at a number of the key elements in clinical research, including: trial design and conduct, clinical research regulations, good clinical practices of research, and the critical evaluation of clinical research in the medical literature. Upon completion, students will have developed essential skills and understanding involving the elements and importance of good clinical research design, implementation, and practices. Clinical and health professionals of all varieties—including physicians, research nurses study coordinators, managers in clinical research and site management, and professionals in the pharmaceutical, biotechnology, and medical device industries, as well as CRO and SMO organizations—will benefit from this opportunity to earn a graduate certificate from Boston University’s School of Medicine, a leading research institution. Why Choose BU’s Graduate Certificate in Clinical Research?
Boston University offers competitive tuition rates that meet the needs of part-time students seeking an affordable education. These rates are substantially lower than those of the traditional, full-time residential programs yet provide access to the same high-quality BU education. To learn more about current tuition rates, visit the Tuition & Fees page . Students can apply a portion of the credits earned in Boston University’s Graduate Certificate in Clinical Research program toward a Master of Science in Clinical Research (MSCR) . (Note: The MSCR program is comprised of campus-based courses and is not available in an online format.) Requirements for the Graduate Certificate in Clinical Research program include an undergraduate degree from an accredited institution and a completed application form. GMSCI640 OL Regulatory and Compliance Issues Graduate Prerequisites: consent of instructor - This course explains the regulatory requirements for health-care products, that is, drugs, biologics, and devices. It is intended for those interested in regulatory affairs or the clinical evaluation, development, manufacture, testing and/or commercialization of these products. Provides an in-depth review of pertinent FDA regulations and guidance and links these to the scientific and logistical activities involved in taking a medical product from research to market. Content and preparation of regulatory submissions, including an Investigational New Drug Application (IND), an Investigational Device Exemption (IDE), a New Drug Application (NDA), a Biologic License Application (BLA), a Pre-Market Approval Application (PMA), and a 510K Pre- Market Notification are described. 4 cr, Fall sem. [4 credits] GMSCI660 OL Good Clinical Practices in Clinical Research Graduate Prerequisites: consent of instructor - This course introduces students to the regulatory responsibilities of sponsors, monitors, and investigators conducting clinical trials. Practical information and exercises are designed to demonstrate GCP compliance from an industry perspective as well as from an academic perspective. Topics Include: Human protection in clinical trials, institutional review boards, selecting and qualifying investigators, consenting subjects, initiating, monitoring and closing out sites successfully and safety monitoring in clinical trials. Group discussions and exercises help students learn practical skills. 4 cr, Fall sem. [4 credits] GMSCI675 OL Designing Clinical Research Studies Graduate Prerequisites: consent of instructor - This course covers important scientific and epidemiologic principles necessary for designing clinical research studies. Topics include bias, confounding, developing the research question, defining an appropriate study population, choosing outcome measures, clinical research ethics and regulation, sample size determination, and statistical analysis issues. Students will design and present a clinical research study during the course. 4 cr, Fall sem. [4 credits] GMSCI789 OL Critical Evaluation of the Medical Literature Graduate Prerequisites: GMSCI675 or Consent of Instructor. - The goal of this course is to provide experience in reading and evaluating the current medical research literature that may be pertinent to the origination, design, implementation, and evaluation of clinical research. In order to most effectively teach these skills, the course will be conducted in a discussion-based format. [4 credits] Getting StartedTo receive more information or to contact an enrollment advisor to start the application process, click the button below and tell us a little about yourself. Someone will be in touch to answer any questions you may have about the program and detail the next steps in earning your degree.
Clinical Research Nursing Certificate Review the online info session here . The Institute for Translational Medicine and Therapeutics (ITMAT) Education program offers an online Clinical Research Nursing Certificate. Through a combination of didactic content, highly interactive and engaging learning experiences, and collaboration with other CRNs, the certificate program will prepare nurses to navigate the growing complexities of the clinical research landscape as well as improve the safety, quality, and experience of research participants. Bridging the gap between clinical care and research operations is an important and timely area of focus for both academic medical centers and study sponsors. The oversight of participants on high risk and early phase research protocols requires expert clinical oversight and coordination of care, and knowledge and experience in research execution. Graduates will have a foundational knowledge of the domains of clinical research nursing including human subjects protection, care coordination and continuity, contribution to science, and study management. Graduates will:
Fall 2024 application submissions are closed. Contact Amanda Brock, Program Director, with any questions: [email protected] Program InformationThe certificate includes 4 courses and is designed to take 1.5 years. Clinical Research Nursing Certificate Study Plan4 Credit Units (CUs)
 Please review the Regulatory Affairs Tuition webpage for details. Tuition and fees are charged per c.u. and certificate program includes a total of 4 c.u. The program is not eligible for federal financial aid. Certificate Eligibility
What We're Looking For
Application ProcessApplicants must submit a full application via the online form in CollegeNET. Please note, select "Regulatory Affairs Certificate" under Program Information as the program of study in CollegeNet. Based on your personal statement response, we will administratively shift your application to the Clinical Research Nursing Certificate. Apply Online Now The application form requires the following documents:
The application does not require GRE score submission. Application Fee: $25 After acceptance, applicants will need to provide proof of RN license and official transcripts. Application SupportWe encourage you to reach out to discuss your interests in the certificate program and answer any questions. Contact Program Director, Amanda Brock, [email protected]. Please review: - The CRN Certificate Handbook - Our Student Resources page . Policies & DisclosuresThe University of Pennsylvania values diversity and seeks talented students, faculty and staff from diverse backgrounds. The University of Pennsylvania does not discriminate on the basis of race, color, sex, sexual orientation, gender identity, religion, creed, national or ethnic origin, citizenship status, age, disability, veteran status or any other legally protected class status in the administration of its admissions, financial aid, educational or athletic programs, or other University-administered programs or in its employment practices. Questions or complaints regarding this policy should be directed to the Executive Director of the Office of Affirmative Action and Equal Opportunity Programs, Sansom Place East, 3600 Chestnut Street, Suite 228, Philadelphia, PA 19104-6106; or 215-898-6993 (Voice) or 215-898-7803 (TDD). Specific questions concerning the accommodation of students with disabilities should be directed to the Office of Student Disabilities Services located at the Learning Resources Center, 3820 Locust Walk, Harnwell College House, Suite 110, 215-573-9235 (voice) or 215-746-6320 (TDD). The federal Jeanne Clery Disclosure of Campus Security Policy and Campus Crime Statistics Act, as amended, requires colleges and universities to provide information related to security policies and procedures and specific statistics for criminal incidents, arrests, and disciplinary referrals to students and employees, and to make the information and statistics available to prospective students and employees upon request. The Campus SaVE Act of 2013 expanded these requirements to include information on and resources related to crimes of interpersonal violence, including dating violence, domestic violence, stalking and sexual assault. Federal law also requires institutions with on-campus housing to share an annual fire report with the campus community. In addition, the Uniform Crime Reporting Act requires Pennsylvania colleges and universities to provide information related to security policies and procedures to students, employees and applicants; to provide certain crime statistics to students and employees; and to make those statistics available to applicants and prospective employees upon request. To review the University’s most recent annual report containing this information, please visit the Annual Security and Fire Safety Report or the Penn Almanac Crime Reports . You may request a paper copy of the report by calling the Office of the Vice President for Public Safety and Superintendent of Penn Police at 215-898-7515 or by emailing [email protected] . Recognizing the challenges of teaching, learning, and assessing academic performance during the global COVID-19 pandemic, Penn’s admissions committees for graduate and professional programs will take the significant disruptions of the COVID-19 outbreak in 2020 into account when reviewing students’ transcripts and other admissions materials as part of their regular practice of performing individualized, holistic reviews of each applicant. In particular, as we review applications now and in the future, we will respect decisions regarding the adoption of Pass/Fail and other grading options during the period of COVID-19 disruptions. An applicant will not be adversely affected in the admissions process if their academic institution implemented a mandatory pass/fail (or similar) system for the term or if the applicant chose to participate in an optional pass/fail (or similar) system for the term. Penn’s longstanding commitment remains to admit graduate and professional student cohorts composed of outstanding individuals who demonstrate the resilience and aptitude to succeed in their academic pursuits. Please review the University of Pennsylvania required disclosures . The University of Pennsylvania is accredited, but there is no separate accreditation for clinical research nursing programs. The Clinical Research Nursing Certificate program does not currently accept students based outside of the U.S. Programs in Clinical Research1 year | full-time, 2 years | part-time, boston university medical campus. Boston University’s programs in Clinical Research aim to meet the needs of health professionals engaged in the full-spectrum of patient-oriented research. We offer both master’s degree and certificate programs to individuals seeking careers in clinical research in industry or academic settings. Clinical Research program offerings:
About the MS in Clinical Research Program (MSCR)Located in Boston’s South End at the Boston University Chobanian & Avedisian School of Medicine, the Master of Science in Clinical Research Program (MSCR) has been in existence since 2001 and is ranked #1 by College Choice among universities that offer an MS in Clinical Research. As an entity of BU CAMED, students are provided with opportunities and are exposed to resources that are part of the Chobanian & Avedisian School of Medicine , the School of Public Health , the Goldman School of Dental Medicine , Boston Medical Center , two VA Administrations, and BioSquare. All of this offers students endless opportunities for personal, academic and professional development. The Master’s in Clinical Research program, which is a STEM program, teaches students the scientific fundamentals of human research. Courses in our curriculum provide an in-depth look at all of the key elements in clinical research, including: trial design, trial management, biostatistics, ethical issues, and clinical research regulations. Other courses cover how basic science discoveries translate into clinical research and new therapies. The total course requirement is 32 credit hours: 22 hours are required curriculum, including practicum and research credits; 10 hours are elective courses. Students will also complete a research “practicum”; a hands-on involvement in a clinical research project under a scientific mentor. The final requirement for the degree is to conduct clinical research, write, and present a capstone project.  Who is this program designed for?The Master’s in Clinical Research program is designed for anyone interested in a career in clinical research. This includes those with MD or PhD degrees interested in becoming independent principal investigators, as well as those with bachelors or masters degrees who seek advancement in a research career in industry or academic settings. Online Graduate Clinical Research CertificateIf you are not ready to commit to our Master’s program, we also offer an Online Graduate Certificate in Clinical Research. This certificate program involves 4 courses across two semesters, and can be completed in one academic year. Learning ObjectivesUpon completion of the Master’s in Clinical Research program, students are expected to:
Our MissionInspire, Instruct, Innovate … The MS in Clinical Research program is dedicated to the discovery, development, and application of knowledge as it pertains to all areas of clinical research. Our mission is to foster an engaging and effective educational environment that promotes the pursuit of outstanding teaching and learning through formal classroom and practical training. With established collaborative relationships with pharmaceutical, biotech, and academic institutions, students are provided with unique opportunities to pursue clinical research in areas that are of personal and professional interest. We hope that the information you receive about our program encourages you to pursue your graduate degree in clinical research with us. If you are interested, click here to get your application started. If you would like to visit our campus or have any questions about our program, please contact Stacey Hess Pino at [email protected] . We look forward to meeting and/or speaking with you! Janice Weinberg, ScD Director, MS in Clinical Research; P rofessor, Department of Biostatistics Stacey Hess Pino, MS, MS Assistant Director, MS in Clinical Research; Director, Online Graduate Certificate Program in Clinical Research; Assistant Professor, Department of Medical Sciences & Education Home > blog > > clinical research course eligibility job and salary Clinical Research: Course, Eligibility, Job and Salary The health industry plays a significant role in the economy. In addition to offering healthcare services, it gives potential students excellent academic and employment options. One such area of study that has grown in popularity recently is clinical research. Download Virohan myCareer App for Paramedical Courses What is Clinical Research all About?Clinical research is a division of the healthcare industry focused on human subjects and medical research. It includes research on drugs, medical technologies, and diagnostic tools that evaluates possible treatments and their impact on people. It also involves clinical trials, enhancing our understanding of the various treatment choices, risk factors, and potential benefits. Clinical Research Course Highlights
What Do Clinical Research Students Do?In clinical research institutions, students learn various medical research aspects. Students study and analyze human health and illness in clinical research. In addition, clinical research students learn a wide range of scientific investigational components, including conducting scientific studies on healthy or diseased human beings. As future clinical researchers, they are trained to create new medical technologies, diagnostic tools, and treatments to treat a wide range of disorders. Also Read: X-ray Technician Course Clinical Research Course DetailsClinical research is the perfect career option for those willing to pursue a career in the health and research sector. Find the information one must know about the clinical research course. Also Read: Paramedical Courses Clinical research (CR) courses are available in
Clinical Research Course Duration
Clinical Research Course FeesThe clinical research course fees vary depending on its level, type, institution, and mode of instruction. According to the level of the courses, the table below lists various clinical research course fees.
Who can opt for the Clinical Research Course
Admission in the Clinical Research CourseMedical and paramedical institutions conduct various entrance tests for the clinical research course at the UG, PG, and PhD levels. The grades a student receives in the 12th standard board and the entrance test scores determine together to determine whether or not the student is eligible for admission to the college. Aspiring students can appear for the following entrance tests for clinical research courses across India:
Check out: Best MBA Colleges in India Clinical Research Course Eligibility CriteriaThe eligibility criteria for a clinical research course vary depending on the institution and course level. Below are the specific eligibility requirements for the different levels of clinical research courses.
Also Read: MBA in Hospital Management Clinical Research Course Structure and CurriculumThe subjects included in a clinical research course's curriculum vary depending on the course type, duration, and institution. The subjects covered by various clinical research courses are listed below.
Check out: General Duty Assistant Course Clinical Research Course Salary and PerksYou can apply for jobs such as clinical research associate, biostatistician, clinical research analyst, clinical supervisor, professor, and more after taking a clinical research course. A list of various clinical research positions and their pay is tabulated below.
Job Opportunities for Clinical ResearchAfter completing a diploma, graduation, or PG in clinical research, you can apply for jobs in various clinics, hospitals, and healthcare businesses. Data management, clinical trials, medical writing, project coordinators, principal investigators, the pharmaceutical industry, and many more fields are all viable employment options. One might choose careers like a bacteriologist, clinical research analyst, clinical research professor, clinical research associate, biostatistician, and more. Also Read: Physician Assistant Course Healthcare Superhero Banein!Virohan ke expert career counsellor se baat karein aur healthcare me progressive career options ke bare me aur jankari paayein! Why should I choose clinical research as a career option? Do clinical researchers earn a good living? What entrance tests are to take to get into a clinical research course? What are the job opportunities after I complete the Clinical Research course? We will get back to you in 2-3 days. Paramedical
Courses after 12th
Medical courses after 12th
Virohan is India's best institute for healthcare and paramedical diploma, advanced diploma, B Voc courses in MLT, OTT, HA, and online certification for working professionals programs which are affiliated with NSDC, IMA, and UGC-RNTU.  Omsk State Medical University
 Omsk State Medical University, Russia : Fees & Admission 2024-25With affordable tuition fee and world-class infrastructure, studying MBBS in Russia has grown to be a top option among medical aspirants around the world. Majority of the Russian medical universities are approved by major international organizations and features top-quality faculties. For Indian students who struggle to get admission in Indian medical institutions, Russian medial universities can be a better option keeping in mind the high-quality of education provided. University at a Glance
Located in the city of Omsk, Russia, the medical faculty at Omsk State Medical University was established in 1920. The university maintains a great relationship with various educational organizations from around the world including countries like Japan, USA, and Western Europe. The clinical departments of Omsk State Medical University are placed in some of the largest municipal hospitals spread across the city, and the hospitals are well-equipped with all kinds of modern tools. Omsk State Medical University features a large library which has a collection of around 6, 00,000 books and publications. With the latest technologies and best teaching methodologies, the university continuously produces the best medical professionals in the world. Accreditation and Recognition of Omsk State Medical UniversityOmsk State Medical University, Russia is rated prestigiously in the world-ranking systems and the MBBS degree they offer are recognized by;
Omsk State Medical University comprises of 59 departments with 73% of the faculty staffs holding an academic degree. Nearly 100 staff members are MD-PhD full professors, while 300 of them are MD-PhD associate professors. Faculties / Departments at Omsk State Medical UniversityOmsk State Medical University, Russia now has 5 faculties as listed below:
Why Study MBBS in Omsk State Medical University, RussiaEver since it became fully functional, Omsk State Medical University has never failed to show up in the list of top 100 universities in Russia. Having trained over 30,000 medical professionals since its advent, the university is renowned for maintaining high-quality educational standards. Every year over 4500 students applies to study MBBS at Omsk State Medical University of which 1200 students hail from nearly 50 foreign countries. When it comes to both accommodation and campus infrastructure, the university is well-equipped with modern facilities. Here are a few benefits you need to know about Omsk State Medical University before making your decision to study MBBS in Russia.
Duration of MBBS in Omsk State Medical UniversityThe duration of MBBS at Omsk State Medical University, Russia is 6 years where the first 5 years are dedicated to theoretical studies and the last one year to clinical practice/ internship. During the second and third year, students can opt to take part in practical researches as well. Intake for MBBS Admission in Omsk State Medical UniversityThe admission for MBBS undergraduate and postgraduate courses at Omsk State Medical University, Russia opens on July and runs till August. However, it is important that you apply before 30th July every year as the visa process can take anywhere from 2-3 months. There were also instances when students were denied a visa due to minor errors. Eligibility for MBBS Admission in Omsk State Medical UniversityIndian students who wish to study Omsk State Medical University, Russia needs to fulfil the following eligibility criteria to quickly get admission.
Documents for MBBS Admission in Omsk State Medical University
MBBS Admission Procedure in Omsk State Medical UniversityAll the candidates who meet the above-mentioned eligibility criteria can easily procure a seat at Omsk State Medical University, Russia. Briefly listed below is the admission security to apply to Omsk State Medical University, Russia.
We assist you throughout the entire process of applying to the university till completing your 6-year MBBS course at the university in numerous ways. We carefully collect, analyse and send your documents to the university on your behalf, and initiate the visa process after receiving the invitation letter. With us, you will always get to enjoy a taste of the trust factor. MBBS Fees in Omsk State Medical University
Disclaimer: Study Europe Consultants cautiously affirm that the given amount of Tuition Fees is not volatile and subject to change without any prior intimation to the students. Also, the amount is in USD, not in Indian Rupees. To know the exact tuition fees, Hostel Fees, Living Cost and Exchange Value of USD in Indian Rupees, please contact our experts at Study Europe. Accommodation Facilities in Omsk State Medical UniversityOmsk State Medical University offers a total of six hostel building for students to reside in of which 3 hostels are specially prepared to accommodate international students. The three dormitories together house around 1344 students on double or triple sharing basis. Every hostel is equipped with facilities like kitchens, bathrooms, study rooms, and shower units. Internet facility is available 24/7 and Indian food is also made available to Indian students in the hostel’s mess. As Omsk State Medical University put special emphasis on the students’ health, the campus also features well-equipped gyms, sports and recreation centers, and ski-centers for students. The university conducts various sports and recreational activities for students every year as Omsk State Medical University strongly believes it helps in turning the candidates into better versions of themselves. Additionally, the creative groups for international, regional, as well as national competitions open a sea of opportunities for the students. Why Students Prefer MBBS Study from Russia?Russia has been the top choice of not only the Indian medical students but also for the medical aspirants from the different corner of the world. Today, Russia has become the education hub. There are many reasons making MBBS in Russia the top choice for medical students. The Top Reasons for MBBS in Russia
MBBS Fees Structure and Course Duration
Why Choose Study Europe.in?
Contact Us for guaranteed admission in the English Medium NMC approved Medical Colleges at affordable cost and study MBBS Georgia | MBBS Russia | MBBS Ukraine and MBBS Kyrgyzstan. Call Us: +91-9289064809 or Write us: [email protected] Visit Our Office: N Block, 2nd Floor, Sector-18, Noida, Uttar Pradesh - 201301 (India) Top Universities in RussiaDownload university brochure, omsk state medical university brochure. REVIEWS AND STUDENTS TESTIMONIALS MBBS Russia Aditi SharmaIt is a great place to study MBBS as the universities are so technologically well equipped. There is so much advancement in the whole education system of Russia.  Niharika SinghI am a student good in study. I was able to fulfill my dream of studying medicine abroad with the support of professional counselors at Study Europe based in Delhi.  My name is Kiran Deep. I wished to pursue MBBS from Russia. Study Europe helped me seek admission in a renowned Russian medical institution.  Pradyuman DeshpandeyMy name is Pradyuman from Ahmedabad. Study Europe provided me the right solution to my concerns and today I am pursuing course in medicine.  Pooja SharmaThe fee structure is quite affordable at Russian MBBS universities which are a great advantage for Indians, as fees in India are quite high. view Photo Gallery  Our Latest BlogRead through the latest blogs to learn more about study abroad essentials,Tuition Fees, Universities, Intake, eligibility, admission process and more.  Review of MBBS Gadget for Indian St..As a medical student, life can be challenging and stressful. With so many books to read and words to recall  An Overview of MBBS in Kazakhstan F..Benefits of Studying MBBS in Kazakhstan, Medical Study in Kazakhstan advantages and disadvantages. For more information contact us now.  Study MBBS In Kazakhstan To Achieve..Kazakhstan is one of the beautiful countries that attract many students every year due to its educational pedagogy. NMC accredits the top-notch medica...  Medical Study in GeorgiaAs per the sources, over 2000 Indian students (approx.) are enrolled for the MBBS Program in the various Georgian medical universities known for high ...
 Omsk State Medical UniversityThe university.
Omsk State Medical University was established in 1920 in the Omsk city of the Russian Federation and is one of the oldest medical universities in Russia. It is also one of the largest as well top-ranked medical universities in Russia, constantly securing its position in the list of 100 best universities in Russia. For the clinical training of the students, the University has affiliations with 33 hospitals and clinics in the city. Students are provided education with state-of-the-art infrastructure, including research labs and modern medical equipment. In a century-long time, more than 35,000 doctors have been trained at Omsk State Medical University. Omsk State Medical University is duly approved by the Medical Council of India (MCI) and offers a 6-Year MBBS Program for local as well as international students. Medical aspirants from India who have qualified NEET can apply for direct admission to the MBBS Program of Omsk State Medical University.  Ministry of Science and Higher Education, Russia  World Directory of Medical Schools (WDOMS)  Medical Council of India (MCI)  Educational Commission for Foreign Medical Graduates (ECFMG)  Medical Council of Canada (MCC)  Foundation for Advancement of International Medical Education and Research (FAIMER) To get admission to the MBBS Program of Omsk State Medical University, the student must qualify NEET-UG (National Eligibility cum Entrance Test-Undergraduate). Besides NEET-UG, there is no requirement to go through any additional entrance examination.
RUS EDUCATION SUPPORT  INDIAN FOOD MODERN CLASSROOMS Medical Laboratories Clinical Training Recreational Facilities Ensured Safety FMGE (Foreign Medical Graduates Examination) Preparation  USMLE (United States Medical Licensing Examination) Preparation 
University AddressMbbs program, admission & support, medical licensing examination support, student life.  In a century-long time, more than 35,000 doctors have been trained at Omsk State Medical University, which is indeed a remarkable achievement. Now, every year, more than 8,500 students graduate from the University. The University has a team of about 500 faculty members. University’s campus is divided into six buildings. For the clinical training of the students, the University has affiliations with 33 hospitals and clinics in the city. Students are provided education with state-of-the-art infrastructure, including research labs and modern medical equipment. Library of Omsk State Medical University is one of the oldest and leading university libraries in the Russian Federation. It houses a collection of more than 6,00,000 books and also provides access to eBooks, online medical journals, and other internet resources. To help the students easily find and borrow books, an electronic system is installed in the library. Students are given access to the library database in Russian as well as the English language. The University is actively involved in various research projects related to medicine and healthcare. Students at Omsk State Medical University get an opportunity to participate in those projects, be a part of the future of medicine, and develop significant skills during the program. Quality of Omsk State Medical University’s education system is also certified by the International Organization for Standardization (ISO). For a personalized and modern learning experience, the University has well-equipped classrooms and follows an apt-student teacher ratio. On the campus, the students also have access to various laboratories, including Physics lab, Chemistry lab, and Physiology lab. The University preserves its 100 years of glorious history and achievements with the help of a dedicated museum on the Campus, which is open for all students and University visitors. Omsk State Medical University is also associated with many medical universities and other institutions in Russia as well as other countries to increase joint initiatives, share resources, and facilitate student exchange programs. Omsk State Medical University is well-recognized in Russia as well as internationally. It is listed in the World Directory of Medical Schools (WDOMS) and certified by the Educational Commission for Foreign Medical Graduates (ECFMG), United States of America. Also, Omsk State Medical University is duly approved by the Medical Council of India (MCI) and the Medical Council of Canada (MCC). Omsk State Medical University offers a 6-Year MBBS Program for local as well as international students. Medical aspirants from India who have qualified NEET can apply for direct admission to the MBBS Program of Omsk State Medical University.  Omsk State Medical University Ulitsa Lenina, 12, Omsk, Omsk Oblast, Russia, 644099  Omsk State Medical University offers a 6-Year MBBS Program in Russian Medium. For international students, classes for initial years may be conducted in English language. The Program for MBBS in Russia is focused on building a strong academic base with a pragmatic approach to education and medical research. To provide hands-on clinical experience, the students studying MBBS in Russia are involved in clinical training from the second year of MBBS. While education in classrooms and laboratories helps the students develop academic skills and sound theoretical understanding, clinical training in University-affiliated hospitals help them apply their knowledge into practice.  To get admission to the MBBS Program of Omsk State Medical University, you can apply online at Rus Education website. Rus Education is exclusively authorized by the Russian Centre for Science and Culture (Cultural Department of The Embassy of the Russian Federation in India) to promote Russian Education among Indian Citizens. Rus Education is also an authorized associate of Omsk State Medical University. We facilitate one-window admission to the MBBS Program of Omsk State Medical University with no requirement of any donation or capitation and without any entrance examination. 
 For the living arrangement of students, Omsk State Medical University maintains 6 dormitories. Hostel facilities are well-furnished and equipped with facilities required for daily student life, including shared kitchens, sports facilities and study space. Two or three students share one hostel room. The University maintains its own sports and recreational facilities, and interested students can play the sports of their choice to relax and stay fit. To arrange recreational activities and community programs for the students, the University has a dedicated Department of Extracurricular and Social Activities. To involve students in social work, University runs different volunteering and charity activities. Depending on their interests, students can also join creative activities like dance, singing, and humor organized by student clubs. For promoting healthy lifestyle and fitness among students, the University has 22 sports clubs and also operates two gyms and five fitness centers on the campus. These facilities open up a wide array of options for the students to play their favorite sports and stay energetic. On off-days, students can also go out to explore Omsk city, which is one of the largest cities in Russia, offering plenty of avenues for amusement. Omsk has numerous museums, historical architectures, classic theaters, and educational institutions that students can explore. While food is available through the mess facility of the University, occasionally, students can go out for dining. To get around in Omsk, students don’t face any problems as the city has well-regulated means for public transport, including buses, taxis, metro, and railways. Being a city where people of many nationalities and religions live together, Omsk offers a high culture and friendly environment for strangers and locals alike. So, Omsk State Medical University and the Omsk city offers the very best of education as well as life, making student life not only fruitful but exciting, fulfilling, and full of experiences, and memories. TOP MEDICAL UNIVERSITIES IN RUSSIA.jpg) Perm State Medical University.jpg) Tver State Medical University.jpg) Orenburg State Medical University Mari State University.jpg) Siberian State Medical UniversityA php error was encountered. Severity: Notice Message: Undefined variable: countries Filename: includes/footer.php Line Number: 26 File: /home/mbbsinrussia/public_html/application/views/includes/footer.php Line: 26 Function: _error_handler File: /home/mbbsinrussia/public_html/application/controllers/University.php Line: 46 Function: view File: /home/mbbsinrussia/public_html/index.php Line: 315 Function: require_once Severity: Warning Message: Invalid argument supplied for foreach() Message: Undefined variable: state Line Number: 44 File: /home/mbbsinrussia/public_html/application/views/includes/footer.php Line: 44 Function: _error_handler ©2024-25 Rus Education. Omsk State Medical University 644099, Ulitsa Lenina, 12, Omsk, Omsk Oblast, RussiaNearest airport: omsk tsentralny airport.  3700 US$/YearStarting fees:. Altai Krai, RussiaCity and province.
About Omsk State Medical UniversityIt was established in 1920 as the medical faculty of the Siberian Institute of Veterinary Medicine and Zoology, and was reorganized in 1921 as the West Siberian State Medical Institute. Omsk State Medical University Russia (OSMU Russia) is the School of Medicine in Omsk, Russia. The university was renamed Omsk State Medical Institute in 1925 and Omsk State Medical Academy in 1994. This medical university has 59 departments. 73% of the total employees have an academic degree. Omsk State Medical Academy has been consistently ranked among the top 100 universities in Russia. Omsk State Medical Academy has received IVth level recognition. In 2015, the academy was granted university status Get Call Back from our MBBS Abroad CounsellorsAffiliation and recognition of omsk state medical university. The Omsk State Medical University has been affiliated and recognized by the various medical council bodies:
 Why Study MBBS At Omsk State Medical University?The reasons why one should pursue MBBS at Omsk State Medical University are as follows:
Quick Highlights Of About Omsk State Medical University
Advantages Of Study MBBS In Omsk State Medical University
Duration Of MBBS In Omsk State Medical University
Omsk State Medical University Fees Structure 2023-24Omsk State Medical University fee structure 2023-24 for MBBS program is shown below:
Faculties of Omsk State Medical University
Omsk State Medical University Ranking 2023-24
Eligibility To Study MBBS In Omsk State Medical UniversityThe following are the eligibility criteria for studying MBBS at Omsk State Medical University:
Omsk State Medical University Admission Process
Document Required For Omsk State Medical University AdmissionThe documents which are required while taking admission at Omsk State Medical University are as given below:
MBBS Syllabus at Omsk State Medical UniversityThe complete syllabus of studying MBBS at Omsk State Medical University are as follows-
Hostel And Accommodation At Omsk State Medical UniversityThe hostel and dining facilities at Omsk State Medical Academy are as follows:
Why MBBS in Russia?These are the following reasons listed below-
Benefits of Study MBBS in Russia
Our Assistance for MBBS Admission in Omsk State Medical UniversityRMC educational service center is a very old and experienced consultancy with having over 10,000 clients. We make sure you get every detail and assistance required for the universities you’re interested in. We bring out the best options based on your budget or affordability, aptitude, subjects, career choices, and many more essential factors. The important part is students can directly apply to Russian universities with RMC. We help you through everything from immigration and visa procedure to booking flights. Youll be guided by experts and be given the right advice and direction. Popular MBBS Destination For Indian Students
Frequently Asked QuestionsThe summer vacation starts from 1st July to 31st August at the Omsk State Medical University. Indian food is available at the hostel. All types of vegetarian and non-vegetarian food are available at the hostel, and in Russia, there is a wide variety of milk products and fruits. At Omsk State Medical University, the teacher student ratio of 110 gives students a more attentive and personal learning environment. The provides facilities such as well-equipped gyms, a sport and recreation. The university provides facilities such as well-equipped gyms, a sport and recreation centre, and ski centres for physical fitness. Omsk State Medical University Address is Ulitsa Lenina, 12, Omsk, Omsk Oblast, Russia,644099. Top Medical Colleges
Philippines
Central America
South America
 TestimonialsTianjin University is a fine university with standard syllabus, homely environment and friendly and talented teachers. The teaching process is based on practical knowledge. Assignments, projects and seminars are frequently assigned to enhance student’s knowledge. I thanks to RMC for all your help. POOJA SINGHAL (AGRA, UP), STUDENT CHINA
Omsk State Medical University Russia 2024-25: Fees, Ranking, Admission, Courses, Eligibility etc. Omsk State Medical University, located in Omsk, Siberia, is a top choice for studying MBBS in Russia. Founded in 1920 as the Medical Faculty of the Siberian Institute of Veterinary Medicine and Zoology, it offers a variety of undergraduate and postgraduate programs in different medical fields, including General Medicine, Pediatrics, Dentistry, Pharmacy, and Preventive Medicine.
This article provides information about Omsk State Medical University, mainly for Indian students, such as Faculties, courses offered, admission process, eligibility criteria, fee structure, Ranking in Russia etc. [Page Index]College summary. Before we complete the college summary, let us look at the major details of Omsk State Medical University Russia . Wants to Study MBBS Abroad from a top Country with low tution Fees? Subscribe Now!
Affiliation and RecognitionThe Omsk State Medical University is one of the biggest medical universities in Russia, and it is affiliated and recognized by various Medical Councils such as:
Omsk State Medical University Consists of five faculties. Here we have listed below:
Courses OfferedOmsk State Medical University Russia Courses offer quality medical programs under highly qualified faculty and state-of-the-art infrastructure. It is famous for its undergraduate medical programs. 
Why study at the Omsk State Medical University Russia?
Admission ProcedureIf you want to take Omsk State Medical University admission to Russia in 2024-25, you must qualify for the National Eligibility Entrance Exam (NEET) for Indian students. Eligibility CriteriaIn this section, all the students check the eligibility criteria of Omsk State Medical University Russia.
Graphical Representation of Eligibility Criteria 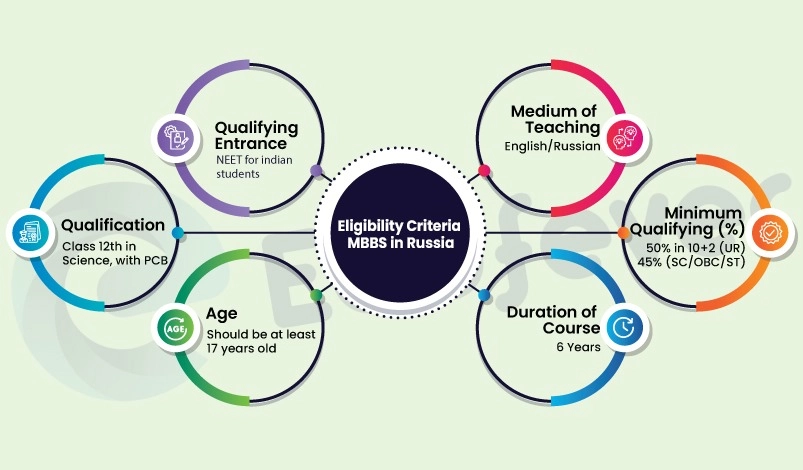 Documents RequiredBefore admission to Omsk State Medical University Russia, please do not forget to carry all these related documents.
Fee Structure 2024-25In this section, all the MBBS students get information about the Omsk State Medical University MBBS fees in 2024. Check all the relevant queries regarding fees following this page: Low fees for MBBS Colleges in Russia .
Ranking 2024-25According to Edurank, the Omsk State Medical University ranking in Russia and all over the world ranking:
Advantages of MBBS in Russia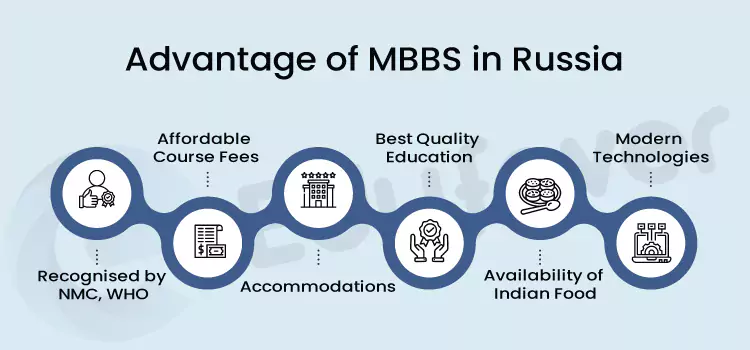 About Omsk City
Temperature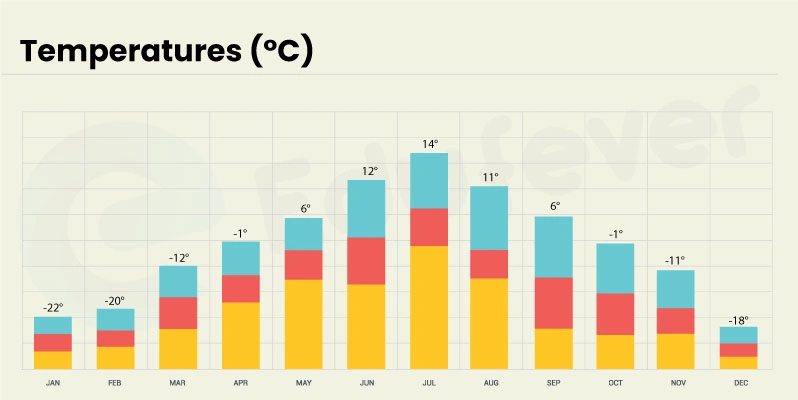 Contact DetailsOmsk State Medical University Russia (OSMU Russia) Address: Ulitsa Lenina, 12, Omsk, Omsk Oblast, Russia, 644099 Omsk State Medical University Photos Frequently Asked Questions (FAQs)When is summer vacation starting at omsk state medical university russia. The summer vacation starts from 1st July to 31st August at the Omsk State Medical University. What type of food is available at the hostel?Indian food is available at the hostel. All types of vegetarian and non-vegetarian food are available at the hostel, and in Russia, there is a wide variety of milk products and fruits. How is the classroom environment at Omsk State Medical University Russia?At Omsk State University, the teacher-student ratio of 1:10 gives students a more attentive and personal learning environment. Which facilities does the university provide for student’s physical fitness?The university provides well-equipped gyms, sports and recreation centres, and physical fitness ski centres.
You may like to read
About HarshHello, I'm Harsh Begwani, with a year of expertise in MBBS and Ayush courses. I have detailed knowledge of various colleges' fee structures, cutoffs, and intake procedures. If you're looking for insights or assistance in pursuing MBBS or BAMS courses, feel free to comment below—I'm here to help! Leave a Comment Cancel replyNotify me via e-mail if anyone answers my comment. Abroad MBBS Update 2024 : Admission Dates, Top College, Fees, Location, Scholarship etc. Get admission to Top Overseas Universities with Affordable Fees.  | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
IMAGES
VIDEO
COMMENTS
Foundations of Clinical Research. This Harvard Medical School six-month, application-based certificate program provides the essential skill sets and fundamental knowledge required to begin or expand your clinical research career. Learn More. September 28, 2024 - April 6, 2025. $6,900 - $7,900.
CCRPS: Clinical Research Training & Certification I Online ...
In summary, here are 10 of our most popular clinical research courses. Data Management for Clinical Research: Vanderbilt University. HI-FIVE: Health Informatics For Innovation, Value & Enrichment (Clinical Perspective): Columbia University. Advanced Clinical Data Science: University of Colorado System. Clinical Data Science: University of ...
Clinical Trials are a key tool in the evaluation of new strategies for prevention and treatment of disease. This certificate program focuses primarily on the design and analysis of randomized clinical trials for evaluation of licensed and non-licensed medical products and other health interventions, the regulatory framework for the conduct and ...
The course textbook, Principles and Practice of Clinical Research, Fourth Edition (2018, ISBN: 978 -12-849905-4) is available for purchase from several online book retailers and at the NIH Building 10 FAES book store. Copies of the textbook can be purchased through Elsevier with a 30% discount and free shipping using the discount code BIOMED30.
The Foundations of Clinical Research program is rooted in the belief that clinical research training is critical to professional development in health care. Clinical research training not only creates potential independent investigators but also enables clinicians to advance their careers through a greater understanding of research evidence.Designed to provide learners with the foundational ...
Learn Clinical Research, earn certificates with paid and free online courses from Harvard, Stanford, University of Michigan, Johns Hopkins and other top universities around the world. Read reviews to decide if a class is right for you.
GMS CI 675 Designing Clinical Research Studies (4 units), fall term. GMS CI 790 Seminar in Clinical Research (2 units), spring term. GMS CI 794/795 Practicum in Clinical Research (2 units), fall or spring term. GMS CI 804/805 Research (2 units), fall or spring term. A minimum of 10 units in elective coursework: A wide variety of courses offered ...
Obtain the skills and knowledge necessary for a career in clinical research. Learn from world-renowned UCSF researchers online, from anywhere. For Institutions & Organizations. Offer online clinical research courses covering a wide array of topics, inlcuding clinical research methods, writing & publication, ethics and mentoring.
Clinical research is the fastest-growing discipline in the burgeoning medical device and pharmaceutical industries. ... visit the Tuition & Fees page. ... ethics and regulation, sample size determination, and statistical analysis issues. Students will design and present a clinical research study during the course. 4 cr, Fall sem. [4 credits ...
Applied Clinical Research Course Fees by Program Year and Semester. Year 1; Year 2; Year 3; Sequence of Courses SCH Fee; Fall 1 (9 SCH) BSCI 5197: Responsible Conduct of Research I: 1 : CTM 5301 : Intro to Principles & Methods of Research: 3 : ACR 5301 : Biostatistics I: 3 : ACR 5104 : Laboratory Rotations: 1: $100:
Master of Science in Clinical Research is a one-year, full-time program with a two-year, part-time option; both options are held in Boston. This in-person program consists of 10 months of didactic activities where time in class is spent discussing the materials and applying it to real-life situations. The courses focus on the fundamentals of clinical research, including the principles and ...
The Foundations of Clinical Research program's six-month online curriculum emphasizes real-time skill-based learning. This approach includes 15 pre-course self-paced online lectures, over 30 hours of live sessions led by faculty of live online-focused symposia, tutorials, and seminars, and three 14-hour mandatory live online weekend intensive workshops.Throughout the program, scholars will ...
How the Course Works • 7 minutes • Preview module. Course Introduction • 5 minutes. Defining the Space • 10 minutes. Research Data Planning 1 • 13 minutes. Research Data Planning 2 • 24 minutes. Approaches to Data Collection • 8 minutes. 1 reading • Total 10 minutes. Course Logistics • 10 minutes.
Integrate new clinical research knowledge and clinical nursing skills; Apply regulations, processes, and management of human subject research, focusing on the unique nursing contributions to clinical trials of drugs and devices; Learn skills and tools required to effectively execute clinical research protocols in various patient populations and ...
The Master's in Clinical Research program, which is a STEM program, teaches students the scientific fundamentals of human research. Courses in our curriculum provide an in-depth look at all of the key elements in clinical research, including: trial design, trial management, biostatistics, ethical issues, and clinical research regulations.
Clinical Research Courses, Eligibility, Fees, Admission ...
The clinical research course fees vary depending on its level, type, institution, and mode of instruction. According to the level of the courses, the table below lists various clinical research course fees. Course: Fees: Certificate in clinical research: Rs.10,000 - Rs.40,000:
Omsk State Medical University, Russia : Fees & Admission 2024-25. With affordable tuition fee and world-class infrastructure, studying MBBS in Russia has grown to be a top option among medical aspirants around the world. Majority of the Russian medical universities are approved by major international organizations and features top-quality ...
Application Requirements. : This information is required for the online application. Curriculum vitae/résumé/list of awards or publications: You will be prompted to upload either your CV or résumé (.doc, .pdf). : Please provide a 250-500 word explanation as to why you would like to be considered for this program.
For the clinical training of the students, the University has affiliations with 33 hospitals and clinics in the city. Students are provided education with state-of-the-art infrastructure, including research labs and modern medical equipment. In a century-long time, more than 35,000 doctors have been trained at Omsk State Medical University.
Bench fees. Some research programmes incur an additional annual charge on top of the tuition fees, often referred to as a bench fee. Bench fees are charged when a programme (or a specific project) incurs extra costs such as those involved with specialist laboratory or field work.
Omsk State Medical University Courses quality medical programs with highly qualified professors and state-of-the-art infrastructure. ... research work, etc. Omsk State Medical University Fees Structure 2023-24. Omsk State Medical University fee structure 2023-24 for MBBS program is shown below: ... Clinical Physiology, Microbiology and Virology ...
In this section, all the MBBS students get information about the Omsk State Medical University MBBS fees in 2024. Check all the relevant queries regarding fees following this page: Low fees for MBBS Colleges in Russia. Particular. Fees in USD (Per-Year) Fees in INR (Per-Year) Tuition Fee. $ 3,700/-.